Creative Biolabs has extensive experience in the detection and analysis of complement systems, including those with special challenges. You can rely on our expertise, experience, and enthusiasm to provide the best service reliably with a fast turnaround time. Here, we provide a colligation-like region (CLR) of C1q isolation protocol by the pepsin digestion method, hoping to serve as a reference to better promote customers' research.
Isolation of the Collagen-Like Region (CLR) of C1q by Pepsin Digestion
The CLR can be obtained by digestion of the purified C1q molecule with pepsin.
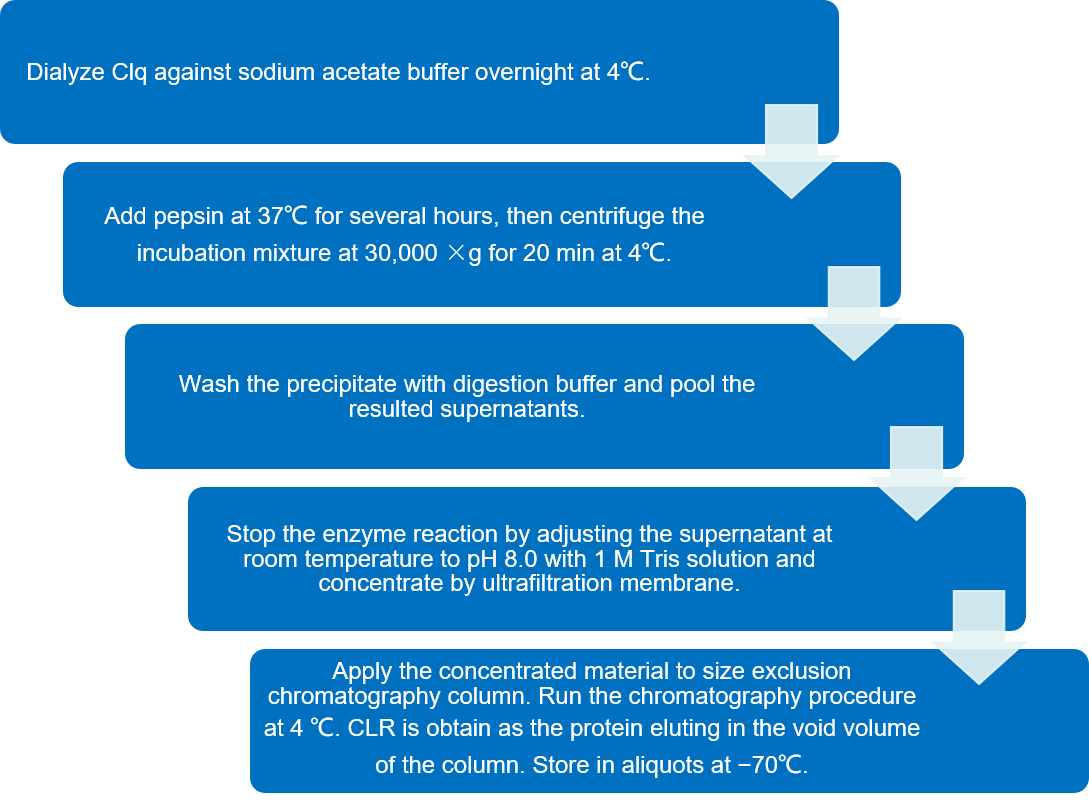
Fig.1 Flow chart of isolation of the CLR of C1q by Pepsin Digestion. (Creative Biolabs)
Published Data
-of-c1q-isolation-protocol-1.jpg) Fig.2 Cleavage of all three chains of C1q-CLR by trypsin.1
Fig.2 Cleavage of all three chains of C1q-CLR by trypsin.1
C1q, a key recognition unit of the classical complement pathway, interacts with cell surface molecules on neutrophils through its collagen-like region (C1q-CLR), activating toxic oxygen species production. To pinpoint the neutrophil receptor interaction site, C1q was isolated, digested with pepsin into C1q-CLR, and further cleaved by trypsin or endoproteinase Lys-C. Functional and structural analyses via gel filtration chromatography revealed that endoproteinase Lys-C cleavage did not impair C1q-CLR’s ability to induce superoxide production in neutrophils. However, trypsin cleavage completely abolished this activity, identifying the interaction site. Circular dichroism confirmed that trypsin cleavage did not disrupt the collagen-like structure, implying loss of activity was not due to denaturation. Irreversible heat denaturation eliminated all activity, emphasizing that the collagen triple helix’s conformation is essential for function. These findings may inform the design of specific therapeutic agents aimed at selectively modulating this response.
Creative Biolabs is a leading provider of customized solutions that specialize in complement-related challenges. We are "complement problem Solvers" focused on providing first-class complement-related services and products. Our services are designed to assist our clients in the discovery and validation of novel complement-related drugs. In addition, we offer a range of customized antibodies, proteins, inhibitors, peptides, and test kits to ensure the success of your specific project. If you would like more details, please do not hesitate to contact us.
Complement Services and Products
-
Complement Products
-
Complement Test Services
Reference
-
Ruiz, Sol, Agnes H. Henschen-Edman, and Andrea J. Tenner. "Localization of the Site on the Complement Component C1q Required for the Stimulation of Neutrophil Superoxide Production (∗)." Journal of Biological Chemistry 270.51 (1995): 30627-30634. Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
Related Sections:


-of-c1q-isolation-protocol-1.jpg) Fig.2 Cleavage of all three chains of C1q-CLR by trypsin.1
Fig.2 Cleavage of all three chains of C1q-CLR by trypsin.1