Complement system is a powerful innate immune mechanism composed by cascades of proteins designed to fight against and abolish intrusion of all foreign pathogens. Here, phagocytosis is known as a central stage in the innate immunity which is triggered by the linkage between ligands on the surface of pathogens and receptors on the membrane of phagocytes. In particular, complement-mediated phagocytosis is achieved by specific recognition on corresponding complement receptors (CRs) such as CR1 (CD35), CR3 (CD11b/CD18, integrin αMβ2, MAC-1), CR4 (CD11c/CD18, integrin αXβ2, p150,95) on the cells.
-
Opsonins and Their Receptors
The main consequence of phagocytosis is the elimination of pathogens. Internal microbes are killed both by toxic reactive oxygen compounds and by microbicidal components (e.g. proteases and lysozyme) on phagocyte granules coupled with the phagosome to form the phagolysosome. Pathogen invasion results in the production of C3 convertase which generates C3b. The end product iC3b binds to several kinds of receptors, CR1, CR3, and CR4. Binding of iC3b to CR3 allows phagocytosis most strongly after phagocyte activation. It is an important resolution of infection and inflammation that CR3-mediated phagocytosis leads to the apoptosis of phagocytic cells.
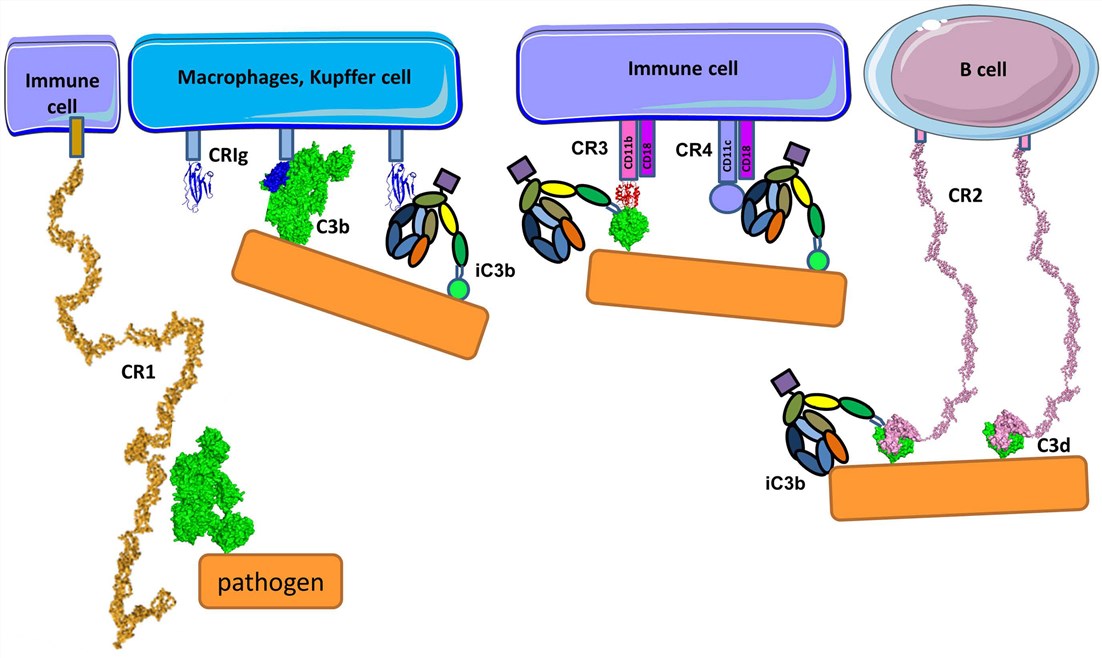
Fig. 1 Complement receptors.1, 3
-
CR1 is a complement component molecule (CCP) domain-containing molecule, implicated in the control of C3 convertases. It’s present on phagocytes, erythrocytes, and kidney glomerular podocytes and can bind C3b and C4b. CR1 on erythrocytes takes a critical part in the clearance of soluble immune complexes, by transmitting them to the liver and the spleen, where they’re removed by macrophages.
-
CR3 is found on monocytes and its expression level increases upon monocyte to macrophage differentiation. CR4 is expressed poorly on monocytes and neutrophils, but its level is up-regulated upon monocyte differentiation to macrophage or to dendritic cells. Among C3 fragments, CR3 and CR4 are specific receptors for iC3b and can induce the phagocytosis of iC3b-coated pathogens. They all belong to the integrin family, involved in the cell adhesion, by virtue of their ability to interact, particularly with intercellular molecule-1 (ICAM-1) on many cells, including endothelial cells. Apart from CR3 and CR4, several resident macrophages (e.g. liver Kupffer cells) express CRIg, a receptor of the immunoglobulin family for iC3b and able to bridge the phagocytosis of iC3b-coated targets.
-
CRs-Mediated Phagocytosis and Measurement
Regulation of CR-mediated phagocytosis is more complicated than simple activation of adhesion within cells. A few decades ago, scientists reported that macrophages can bind complement-opsonized targets but can't phagocytose them without an attached signal. Thus, activation of integrins to be adhesive isn’t enough to make them complete phagocytosis. Though CRs are not generally mobile in the plane of the membrane of unactivated cells, this mobility is required for phagocytosis. Medicines and cytokines that activate CR mobility can also activate complement-mediated phagocytosis. With the modern understanding of integrin function, the data suggested that the resting macrophages in assays likely expressed CRs that were of sufficient affinity to bind opsonized molecules but weren’t capable of the cluster. And cell activation permitted the engaged receptors to cluster, which is necessary for phagocytosis.
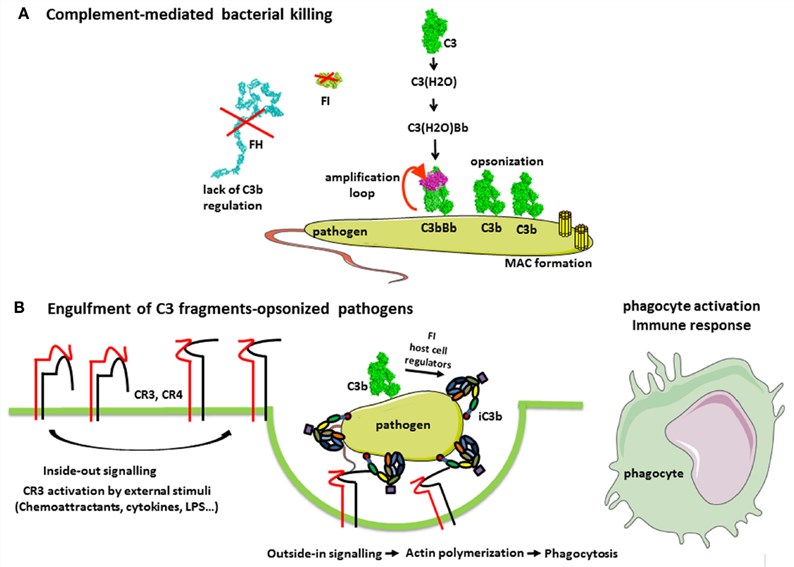
Fig. 2 Complement in the defense against pathogens.2, 3
To figure out the molecular mechanism of phagocytosis, it’s important to establish a reproducible experiment workflow of phagocytosis in vitro. As for Fcg receptor-mediated phagocytosis, erythrocytes or zymosan are utilized as particles to be incorporated and pretreated with specialized antibodies before phagocytosis assays. In the context of complement-mediated phagocytosis, the complement activation cascade in the serum is effectively considered. C3b is naturally produced and destroyed, and when non-self microorganisms are exposed to the serum, C3b binds to them covalently and then becomes C3 convertase by the action of cofactors. Cleavage of C3 to C3b and ultimately to iC3b is amplified and the organism is surrounded by iC3b and contained effectively by phagocytes which express CR3.
Creative Biolabs is a leading biological company that has established comprehensive one-stop services for Complement Therapeutics. If you’d like to know more information, please directly contact us and consult our technical supports online.
References
-
Merle, Nicolas S., et al. "Complement system part II: role in immunity." Frontiers in immunology 6 (2015): 257.
-
Merle, Nicolas S., et al. "Complement system part I–molecular mechanisms of activation and regulation." Frontiers in immunology 6 (2015): 262.
-
under Open Access license CC BY 4.0, without modification
Related Product
For Research Use Only.
Related Sections:


