The complement system actively modulates various steps of an inflammatory response. Currently, inflammation is thought of a complex pathophysiologic process that participates literally hundreds of mediators and distinct cell types and tissues and could be initiated by any stimulus causing cell injury. Inflammation is often a response to infection. Nevertheless, physical or chemical injury alone can also result in this reaction. The final objective of the process is to eliminate the pathogenic agent with minimal destruction to host tissues and to repair the damage induced by initiating factors.
-
Complement-mediated Inflammation Introduction
The complement system was primarily viewed as a “complement” to humoral immunity, and nowadays is treated as a major component of innate immunity, protecting the host from pathogens, coordinating many events during inflammation, and mediating innate and adaptive immunity. Complement is an assembly of proteins identified in the blood, body fluid, and on the cell surface.
Soluble complement constitutes form the proteolytic cascade, whose activation generates complement effectors that target various cells engaged in the immune reaction. Membrane-bound receptors and regulators deliver signals from complement effectors to target cells and restrict complement activation to the surface of pathogens and impaired or activated host cells. The network of multiple interconnections among complement proteins, immune cells, and mediators offers an outstanding mechanism to defend the organism against infections and support the repair of damaged hosts. But interferences in this defense contribute to the pathogenesis of diverse diseases. And the role of complement in different inflammatory disorders is multifaceted.
-
Anaphylatoxins and Their Receptors
Complement anaphylatoxins C3a, C4a, and C5a, are highly relevant, pleiotropic, proinflammatory molecules ~9 kDa in size (74-77aa) created as products of proteolytic activation of C3, complement factors C4, and C5 by upstream C3 convertase, activated C1s, and C5 convertase, respectively. Anaphylatoxins are evolutionarily associated with one another and thus share a relatively high level of homology, as well as somewhat overlapping functions in the generation of immune responses. C5a has been testified to be pretty more potent than C3a and C4a in inducing biological activities, and C4a is the weakest to the extent that physiologically related functions.
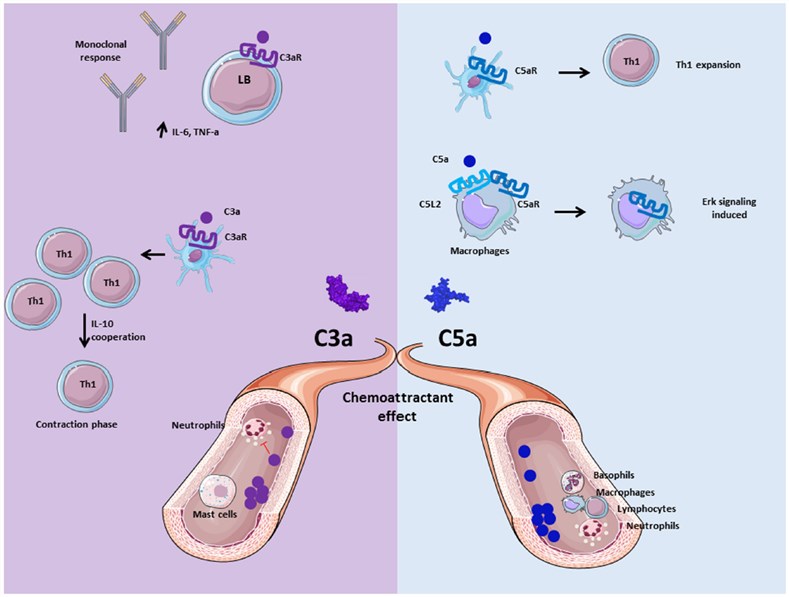
Fig. 1 Role of anaphylatoxins C3a and C5a.1, 2
Anaphylatoxin molecules C3a and C5a play a key role in the regulation of immune system activity by complement. Both two contribute to the inflammation and activate immune cells, non-myeloid cells, which express anaphylatoxin receptors C3aR and C5aR. Human C3a can specifically bind its receptor C3aR, which is a member of the G-protein coupled receptor (GPCR) family with 7-transmembrane domains. Human and mouse C5a bind to C5aR, which also belongs to the GPCR family.
Anaphylatoxins stimulate inflammation by evoking an oxidative burst in immune cells, including macrophages, neutrophils, eosinophils, and basophils. Their physiological functions involve many hallmark proinflammatory reactions, such as leukocyte recruitment, smooth muscle contraction, increases in vascular permeability and in other accouterments of white-blood-cell responses, and facilitating the production of other inflammatory mediators. The C4a seems to have a functional activity on monocytes and macrophages, while a cognate receptor has yet to be reported for this molecule.
-
Crosstalk Between C3aR and C5aR Pathways with Toll-Like Receptor (TLR) Signaling
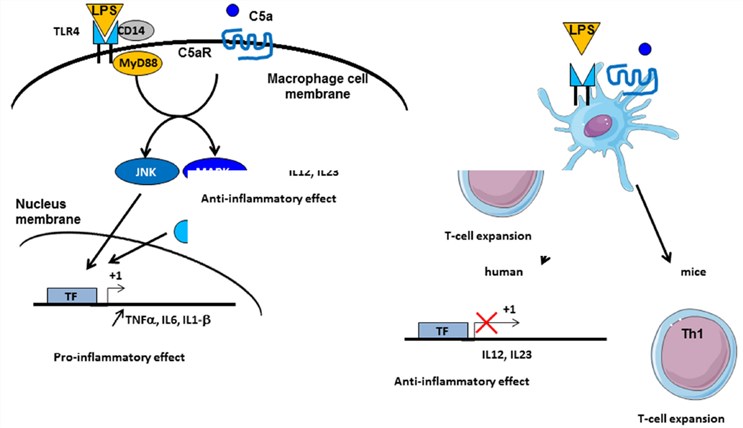
Fig. 2 Crosstalk between complement and TLR pathways.1, 2
Anaphylatoxin C3a and C5a are capable of initiating potent inflammatory pathways through their receptors C3aR and C5aR. The implication of intermediates such as nuclear factor kappa B (NF-κB), mitogen-activated protein kinase (MAPK), and c-Jun N-terminal kinase (JNK) in their transduction implies possible crosstalk with other signaling pathways, such as those of TLRs. Indeed, complement systme takes part in TLR-induced inflammation. Co-activation of MyD88-dependent TLRs (including TLR-2, TLR-4, and TLR-9) and complement in knockout mice improve plasma inflammatory cytokines, including TNF-α, IL-6, and IL-1β. Furthermore, complement activation by the fluid phase cobra venom factor (CVF) synergizes with lipopolysaccharide(LPS) to bring a significant increase of IL-6 production. These results suggest a strong interaction between TLRs and in vivo complement signaling to promote inflammation and coordinate adaptive immunity.
Complement is likely to interact with TLR signaling by C3aR and C5aR, due to the engagement of MAPK, JNK, and extracellular signal-regulated kinase (ERK1/2), but not p38 MAPK. TLR2 can also crosstalk with CR3, since TLR2 will transactivate CR3 by PI3K through an inside-out signal. In reverse, CR3 regulates TLR2 signalization by recruiting TIRAP and helping the recruitment of MyD88 signaling adaptors to initiate further actions.
Creative Biolabs is a leading biological company that has established comprehensive one-stop services for Complement Therapeutics. If you’d like to know more information, please directly contact us and consult our technical supports online.
References
-
Merle, Nicolas S., et al. "Complement system part II: role in immunity." Frontiers in immunology 6 (2015): 257.
-
under Open Access license CC BY 4.0, without modification
Related Product
For Research Use Only.
Related Sections:


