Potency Evaluation Service of Viral Vector
Creative Biolabs provides advanced, customized, and globally compliant viral vector potency assessment solutions. Our services are designed to address the inherent complexity and variability of novel gene therapy products, providing highly sensitive and reproducible data that meets the most stringent quality standards required for commercialization. We are committed to working with you to accelerate your R&D process and reduce regulatory risks.
Viral Vector Introduction
The Cornerstone of Gene Therapy Success
Viral vectors—such as adeno-associated viruses (AAV), lentiviruses, and adenoviruses—are among the most transformative technologies in modern medicine and are key delivery vectors for gene and cell therapies. The success of your candidate therapy from the laboratory to the clinic depends directly on the quality and functional properties of the vector used.
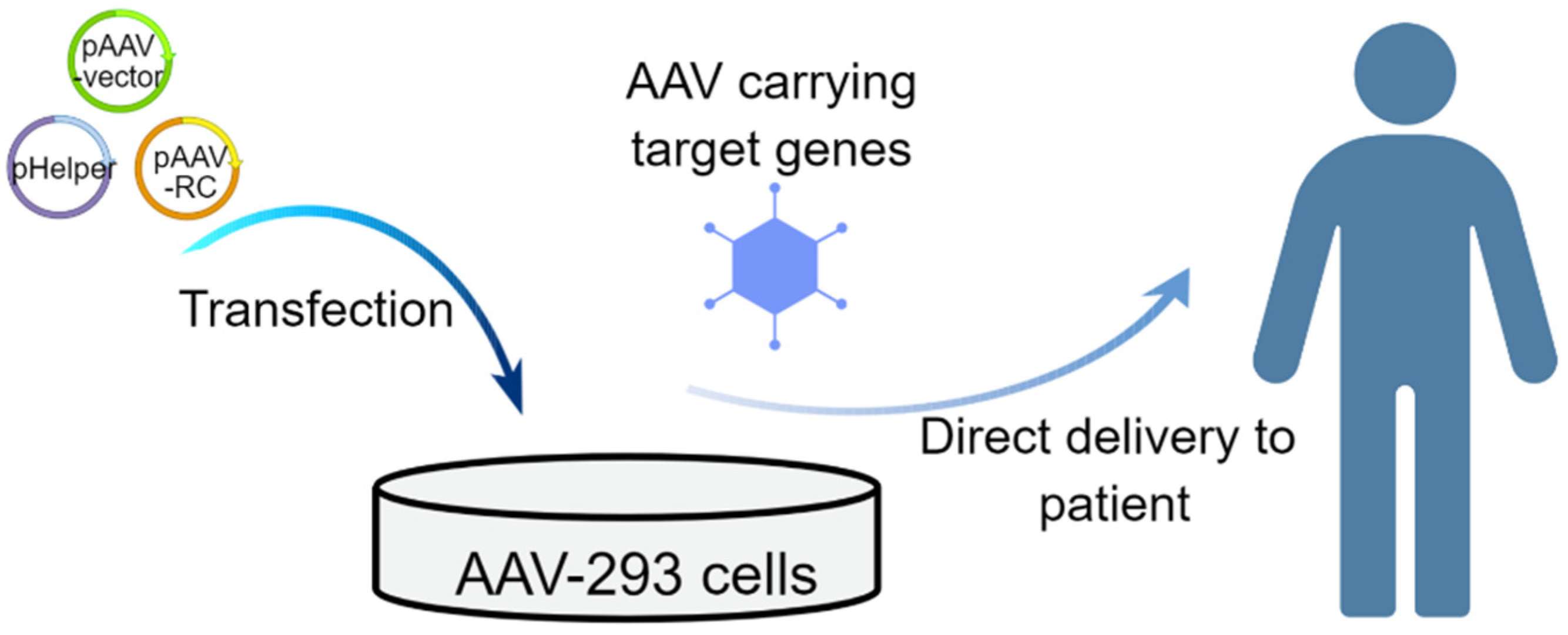 Figure 1. Schematic of the in vivo strategies that use AAV vectors for treating genetic diseases.1
Figure 1. Schematic of the in vivo strategies that use AAV vectors for treating genetic diseases.1
Potentiality is Crucial:
Accurately assessing the biopotency of viral vectors is not merely a quality control step, but a fundamental requirement for successfully conducting preclinical studies, ensuring production consistency (batch-to-batch release), and smoothly completing complex regulatory submissions. Potential refers to a measure of the functional activity of a vector—its ability to induce the desired biological effect (e.g., therapeutic gene expression or cell modification).
Classification and Characteristics
Viral vectors are classified according to their basic biological characteristics. Each type of virus has unique advantages to meet specific research or therapeutic needs:
Adenovirus (Ad)
- Large double-stranded DNA genome with high cloning capacity (up to 36 kb)
- Efficient transduction in both dividing and non-dividing cells
- Transient transgenic expression without genome integration
- Strong immunogenicity, suitable for vaccine applications
- Broad tissue tropism, with natural affinity for respiratory and liver tissues
Adeno-associated Virus (AAV)
- Small single-stranded DNA genome with limited drug loading capacity
- Non-pathogenic with excellent safety profile
- Long-term transgenic expression in non-dividing cells
- Multiple serotypes for tissue-specific targeting (AAV1-AAV9)
- Lower immunogenicity compared to other viral vectors
Retroviruses (including lentiviruses)
- RNA Genome, reversibly transcribed and integrated into host chromatin
- Stable, long-term transgenic expression achieved through genome integration
- Exhibiting broad tissue tropism, lentiviruses can transduce non-dividing cells
- Persistent modification of daughter cells achieved through mitotic inheritance
- Precise integration control achieved through self-inactivation (SIN) design
Potency Evaluation of Viral Vector
Potency assessment, a key quality attribute (CQA) in viral vector characterization, is defined as a quantitative measurement of the vector's mechanism-of-action-specific biological activity. Unlike simple physical titer measurements, potency assays functionally assess the vector's ability to exert its intended biological activity, directly impacting product quality and clinical efficacy. The fundamental principle of potency assessment is to establish a quantitative relationship between vector administration and associated biological effects. This requires carefully designed assay systems that capture key aspects of the vector's mechanism of action while exhibiting sufficient precision, accuracy, and reproducibility to meet batch release decisions. According to emerging international standards, potency measurements must be appropriate for the intended use, statistically validated, and indicative of stability to provide meaningful data throughout the product lifecycle.
Core Services at Creative Biolabs
Vector Copy Number (VCN)
In gene therapy, achieving the desired level of transgene expression is crucial for therapeutic efficacy. Monitoring the vector VCN helps ensure that the appropriate genetic dose is present in the target cells.
We utilize both qPCR and Digital PCR (dPCR) to determine VCN. dPCR is a robust method for accurately measuring nucleic acid targets with precision and reproducibility, eliminating the need for a standard curve or reference to interpret overall quantity.
Empty Particle to Full Particle Ratio
In the process of viral vector preparation, except for the complete viral vector containing a full-length DNA genome, a certain percentage of viral particles do not contain any DNA genome, which is called empty viral particles.
We apply a range of methods, including cryo-transmission electron microscopy (cryo-TEM), volume exclusion chromatography (SEC), and analytical ultracentrifugation (AUC), to efficiently determine total and infectious particle counts.
Infection Efficiency Test In Vitro Expression
Infection efficiency testing typically involves incubating viral vectors with target cells in vitro and monitoring the expression of the protein of interest using various methods, such as immunofluorescence staining or flow cytometry.
Supported Viral Vector Types
We support potency evaluation for all preclinical vector systems, each with its unique analytical considerations:
- Adeno-associated virus (AAV): Detection is highly complex due to the presence of an empty capsid. Our assays are designed to specifically measure functional titers, effectively distinguishing between active and non-functional viral particles containing genes.
- Lentiviruses: Detection is required to confirm stable integration into the host genome for long-term expression. We typically combine potency readings with single-cell analysis to ensure a true reflection of function.
- Adenoviruses: Detection is typically required to measure lysis function or transient expression efficiency due to the rapid turnover required by the viral replication cycle.
- Retroviruses: Similar to lentiviruses, stable integration and expression need to be confirmed.
- Other Novel Vector Systems: We provide consulting and custom method development services for emerging non-viral vectors and novel delivery systems.
Why Choose Our Service?
The success of your advanced therapeutic protocols hinges on the reliability of your vector characterisation data. Choosing Creative Biolabs means partnering with experts who understand the science and regulatory landscape of gene therapy.
-
PhD-level Expertise
Our analytical team comprises experienced virologists, molecular biologists, and bioinformatics specialists.
-
Orthogonal Methodology Strategy
We utilise multiple independent analytical platforms (e.g., sequencing, mass spectrometry, chromatography) to validate critical parameters.
-
Rapid Turnaround and Data Integrity
Our streamlined workflow and dedicated high-throughput sequencing (HTS) infrastructure ensure rapid delivery of high-quality data.
-
Cutting-edge Technology
We invest in cutting-edge platforms (NGS, dPCR, HPLC, MS) to deliver data with the highest precision and sensitivity.
-
Regulatory Support
Our solutions are designed to comply with global regulatory standards and provide comprehensive documentation support for your applications.
-
Customized Solutions
We understand that every vector is unique. We will work closely with you to tailor an authentication strategy based on your specific structure and development stage.
Collaboration Process
Our streamlined five-step collaboration process ensures a clear and efficient workflow from project initiation to final report delivery.
Needs Communication and Exploration
A dedicated scientific liaison will work closely with your team to gain a deep understanding of your project's therapeutic goals, mechanism of action, vector characteristics, regulatory phase, and specific Quality Tolerance Plan (QTP).
Scientific Protocol Design and Pricing
Our senior scientists will develop a detailed and tailored potency assessment protocol, including the testing platform, key reagents, acceptance criteria, and a transparent project pricing.
Method Development and Full Validation
Once the protocol is approved, we will develop the method and, where necessary, conduct full validation in our controlled laboratory environment to determine the system's suitability and performance characteristics.
Sample Testing and Analysis
We will rigorously test your vector samples using validated methods according to Standard Operating Procedures (SOPs).
Report Readiness Delivery
We provide a comprehensive final report and raw data package, including explanations, a summary of method performance, and specific recommendations for future development or regulatory submissions.
Delivery and Support
We are committed not only to generating data, but also to delivering documentation that meets regulatory review requirements.
A well-structured and comprehensive document covering the following:
- Results summary.
- Detailed materials and methods (protocol summary).
- Complete method validation parameters and performance data (if applicable).
- Dose-response graphs and statistical analysis.
- Discussion and interpretation of results in accordance with acceptance criteria.
Customer Review
"We commissioned Creative Biolabs to evaluate the potency of our AAV gene therapy candidate. Their team demonstrated exceptional scientific rigor, developing a mechanism-based potency assay that met our scientific and regulatory requirements. Their comprehensive approach identified key quality attributes we had not previously considered, significantly enhancing our CMC (Chemistry, Manufacturing, and Controls) protocol."
– Director of Process Development, a biotechnology company
"The complexity of our lentiviral vector potency requirements necessitated a sophisticated approach integrating multiple analytical methods. Creative Biolabs provided a complete solution, including vector genomic titers, infection titers, transduction efficiency, and assessment of functional activity in clinically relevant primary cells. Their team worked closely with our scientists to optimize the assay conditions for our specific vector system, ultimately resulting in a robust and reproducible method."
– Head of Analytical Development, a pharmaceutical company
Frequently Asked Questions
Q: What is the main difference between potency assessment and physical titer (vector genome titer)?
A: Physical titer (e.g., GC/mL, determined by qPCR) measures the total number of viral particles, regardless of their functional status. Potency assessment (potency assay), on the other hand, specifically measures the proportion of biologically active viral particles—those capable of successfully infecting target cells and expressing therapeutic genes. For regulatory purposes (IND/BLA), potency is a more critical quality attribute because it reflects the actual therapeutic function of the product.
Q: How does your potency assay design address the issue of empty capsids in AAVs?
A: The presence of empty capsids is a known challenge for AAVs. Our cell-based potency assay is designed to achieve functional selectivity. By measuring the downstream biological effects (e.g., transgene expression) after successful transduction of the vector into cells, our assay accurately quantifies the number of active, intact capsid vectors, thus avoiding interference from non-functional empty vector particles.
Q: Can we use our proprietary cell lines for potency assessment? Can we perform co-culture testing?
A: Absolutely. Using cell lines most relevant to the intended biological function of your product is crucial. Our customized services include the transfer, validation, and verification of assessment methods to specific cell lines provided by our clients. We also possess expertise in developing sophisticated co-culture or primary cell assay methods that better mimic the in vivo environment.
Q: Does your potency testing service include vector stability testing?
A: Yes, we integrate potency testing into comprehensive stability studies. We can assess the functional stability of vectors under various stress conditions (e.g., temperature, freeze-thaw cycles) and long-term storage conditions to determine the appropriate shelf life and handling requirements for your drug.
Connect with Us Anytime!
Leveraging our vast expertise and cutting-edge technology, Creative Biolabs presents comprehensive characterization programs aimed at evaluating the potency of viral vectors, guaranteeing a top-tier product for your needs. Reach out to us for further details regarding viral vector potency testing, and we'll gladly provide assistance tailored to your requirements.
Reference
- Li X, Le Y, Zhang Z, et al. Viral vector-based gene therapy. International journal of molecular sciences, 2023, 24(9): 7736. https://doi.org/10.3390/ijms24097736 (Distributed under Open Access license CC BY 4.0, without modification.)
