Titer Detection Service of Viral Vector
The most important property of viral vectors is infectivity. Assessing infectivity involves examining viral replication within cells to determine the titer of a specific virus stock. Titer denotes the concentration of infectious viral units per specified volume. This calculation serves as a crucial quality control measure in virus manufacturing, ensuring that viral products fulfill customer specifications. As a prominent provider of viral vectors, Creative Biolabs categorizes viral titers into physical and biological titers.
Introduction to Viral Vector Titer
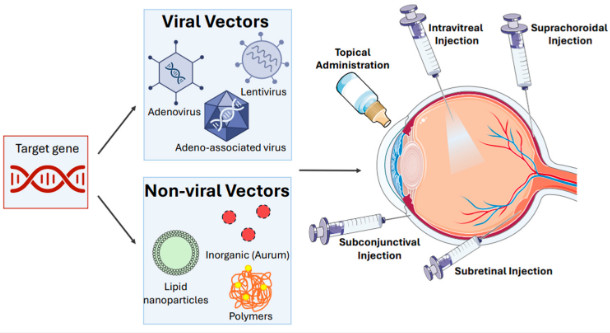 Figure 1 Types of targeted gene delivery for gene therapy of ocular diseases.1
Figure 1 Types of targeted gene delivery for gene therapy of ocular diseases.1
Physical titer quantifies the total number of both active and inactive virus particles, typically expressed as the number of virus particles per milliliter (VP/mL) or for AAV, as the number of genomic copies per milliliter (GC/mL). This includes viral particle titer (or capsid titer) and genomic titer. Physical titer can be determined through quantitative PCR to ascertain the genomic copy number of viral particles, or by assessing specific proteins within the viral particles.
Biological titer involves a series of dilutions of the virus infecting experimental cells, with positivity determined by detecting the expression of the target gene (or transgene). It is calculated based on the number of positive cells or wells, in conjunction with the viral dilution, and is quantified in transduction units (TU). For lentiviral or retroviral vectors, biological titer is denoted as transduction units per milliliter (TU/mL). Adenovirus vector titers are typically expressed as plaque-forming units per milliliter (PFU/mL) or infectious units per milliliter (IFU/mL).
A Flexible Suite of Services at Creative Biolabs
Various techniques are utilized for titer detection of different viruses, reflecting differences in virus biological traits, detection sensitivity, sample types, laboratory equipment, and cost considerations. Creative Biolabs is dedicated to providing top-tier titer testing services for a wide range of viral vectors.
- Lentiviral Vectors Titration
- Adenovirus Vector Titration
- Adeno-associated Virus Titration
- Plaque-Forming Titer Assays
Why Need Titer Detection?
Accurate potency determination is not only a regulatory requirement but also a fundamental element throughout the entire therapeutic drug development lifecycle. In early research stages, accurate quantitative analysis enables meaningful comparisons of different carrier formulations, guiding carrier engineering and process development. During manufacturing, reliable potency determination is crucial for identifying key process parameters, defining appropriate dosages, and ensuring consistency across different production batches.
Our titer testing services are specifically applied in the following areas:
- Cell and Gene Therapy Development: Providing dosage information and validating product consistency for viral vector-based therapies through titer testing, thus supporting their development.
- Vaccine Development: Achieving precise quantification of viral vectors used as vaccine platforms, supporting preclinical and clinical development.
- Process Development and Optimization: Providing critical data to guide upstream and downstream process development, improving yield and product quality.
- Quality Control and Batch Release: Providing validation methods that meet quality requirements for product characterization and batch release testing.
- Stability Studies: Monitoring changes in vector titer over time to determine appropriate storage conditions and shelf life.
Our Technology Platform for Titer Detection of Viral Vector
We are dedicated to providing diverse titer testing methods, ensuring reliable and high-quality viral vectors, as shown in Tab.1.
Tab.1 Test methods of the viral vector titer.
| Test Items | Methods | Description |
|---|---|---|
| Physical titer | Quantitative PCR (qPCR) | The most commonly utilized approach for assessing viral genomic titer. It is characterized by its simplicity, convenience in operation, and notable advantages in quantitative accuracy, sensitivity, and stability. |
| Droplet digital PCR (ddPCR) | This is a highly precise technique that eliminates the necessity for standard curves or high amplification efficiency. It employs primers and probes designed to target the ITR elements within viral vectors, with the flexibility to adjust targeting to alternative sequences if needed. | |
| ELISA |
The determination of viral particle titers involves assessing color development through enzyme-catalyzed substrate reactions linked to capsid-specific binding antibodies. Such as p24 ELISA is used for lentiviral titer testing. |
|
| Fluorescence-Activated Cell Sorting (FACS) |
FACS is applied to viral vectors encoding fluorescent proteins. This method is employed to evaluate the virus's ability to induce transgene expression accurately in target cells. |
|
| Biological titer | Viral plaque assay |
The viral plaque assay stands as one of the most widely adopted techniques for determining infection titer. A permissive cell line is exposed to virus dilutions at low MOI, leading to virus replication, cell lysis, and plaque formation. The number of plaques counted determines PFU/mL. |
| Immunoassay | Immunoassay provide a quick, complete system to functionally titer virus infectivity. | |
| TCID50 | The TCID50 method quantifies the infectious virus titer by determining the amount of virus needed to induce cytopathic effects or cause cell death in 50% of host cells. |
Our Collaboration Process
Our services are designed to integrate into our clients' drug development lifecycle seamlessly:
-
Consultation and Test Design
Our team of PhD-level scientists will conduct an in-depth initial consultation with you to understand the vector type, manufacturing matrix, and development stage. -
Sample Receiving and Quality Control
Rigorous sample integrity checks and pretreatment, including optimized nucleic acid extraction methods for complex bioprocess intermediates (e.g., crude lysis buffers, purified drugs) to minimize inhibition. -
Advanced Titer Analysis
Selected tests (ddPCR, infectiousness tests, ELISA, etc.) are performed according to stringent quality control (QC) standards on our validated, advanced platform. -
Data Analysis and Scientific Reporting
We provide a comprehensive, auditable report containing raw data, calculated titer values, CQA indicators, and a detailed interpretation of the results.
Why Choose Our Services?
Creative Biolabs is unique in its scientific excellence and precise operations:
- Scientific Expertise: Our team comprises scientists with PhDs who possess deep expertise in virology, molecular biology, and regulatory science, ensuring intelligent test design and data interpretation.
- High Throughput and Speed: Utilizing advanced automation and ddPCR technology, we can process large volumes of samples rapidly and synchronously, minimizing manual processing time.
- Orthogonal Validation: We advocate for and provide orthogonal tests (such as ddPCR and infectiousness assays) to comprehensively evaluate vector quality and meet stringent regulatory requirements.
Customer Review
"Throughout our lentiviral vector development program, we have collaborated with Creative Biolabs on titer assays. Their ddPCR method provides highly reproducible data, crucial for process optimization, and has withstood rigorous regulatory scrutiny. The team's deep understanding of viral vector biology and analytical validation has significantly improved our development efficiency."
– Dr. Elena V, Director of Vector Core Facility.
"As our AAV-based gene therapy candidate enters clinical development, we need reliable potency assays for batch release and stability monitoring. Creative Biolabs has developed a suitable assay that meets our time constraints and regulatory requirements. Their method has demonstrated exceptional accuracy and reliability across multiple analysts and instruments, giving us confidence in our product characterization data."
– Prof. David M, Senior Director of Process Development.
Frequently Asked Questions
Q: What is the typical turnaround time for titer assays?
A: For standard physical titer assays using ddPCR, we typically deliver results within 3-5 business days of receiving the sample. Functional titer assessments usually require cell-based assays, which generally take 7-10 business days due to the culture time required. We offer expedited services for time-sensitive projects.
Q: How do I choose the most suitable titer assay method for my viral vector?
A: The best method depends on your vector type, development stage, and specific data requirements. Physical titer assays (ddPCR, ELISA) provide information on viral particle concentration, while functional assays (FACS, TCID50) assess biological activity. In the initial consultation, we will consider your specific needs and recommend the most suitable analytical strategy that balances scientific rigor with practical application.
Q: What sample requirements and shipping conditions do you recommend for our products?
A: We typically require 100-500 µL of appropriately concentrated viral vector samples for testing. For physical titer assessments, samples should be transported frozen on dry ice. In contrast, functional titer assays typically require transport using wet ice or refrigerated packages to maintain their infectiousness. Our team provides detailed transport instructions at project initiation to ensure sample integrity.
Q: Can you develop custom titer assays for novel viral vectors?
A: Absolutely. Our custom assay development services are designed for novel vector systems that standard methods may not adequately handle. We have extensive experience developing and validating suitable assays for emerging vector platforms, incorporating appropriate controls and validation parameters to ensure data reliability.
Q: What are the differences between digital PCR and traditional qPCR in viral vector titer assays?
A: Digital PCR (ddPCR) provides absolute quantification without relying on standard curves, exhibits greater tolerance to PCR inhibitors, and offers higher accuracy even at low target concentrations. These advantages make it particularly important in critical applications where accuracy and reproducibility are extremely important, such as batch release testing and stability studies.
Connect with Us Anytime!
At Creative Biolabs, we understand that accurate potency assessment is fundamental to ensuring the safety, efficacy, and consistency of viral vector products throughout their development lifecycle. We have established a comprehensive infrastructure for quantitative analysis of viral vectors, employing advanced methodologies that surpass traditional approaches in accuracy, reproducibility, and regulatory compliance. Our services cover the entire R&D process, from early-stage research to process development, batch release testing, and stability monitoring, providing critical data to inform decision-making and improve R&D efficiency. Contact us today for a quotation or any question.
Reference
- Kharisova C B, Kitaeva K V, Solovyeva V V, et al. Looking to the Future of Viral Vectors in Ocular Gene Therapy: Clinical Review. Biomedicines, 2025, 13(2): 365. https://doi.org/10.3390/biomedicines13020365 (Distributed under Open Access license CC BY 4.0, without modification.)
