CFI Structure CFI Functions CFI Test CFI Deficiency Therapeutic Target
Complement component factor I (CFI) plays a key role in regulating the complement system. The complement system consists of a variety of proteins that work together to protect the body from pathogens, remove damaged cells, and promote immune responses. Specifically, Factor I acts as a regulator of complement activation by preventing uncontrolled amplification of the complement cascade.
Structure of Complement Factor I
CFI acts as a key regulatory enzyme of the complement system and immunologically maintains system homeostasis by precisely regulating the C3b and C4b gaps. The unique molecular structure and functional properties play a key role between defense and protection of stem cells.
Molecular Structure
CFI is a serine protease encoded by the CFI gene on chromosome 4. It circulates as a disulfide-linked heterodimer comprising a heavy chain (51 kDa) and a light chain (37 kDa). The heavy chain contains four distinct domains:
-
FIMAC domain: Involved in substrate recognition.
-
CD5/SRCR domain: Mediates interactions with cofactors.
-
LDLr1 and LDLr2 domains: Calcium-binding sites critical for structural stability. The light chain houses the trypsin-like serine protease domain, which harbors the catalytic triad (His-362, Asp-411, Ser-507) responsible for cleaving C3b and C4b.
The light chain contains the serine protease catalytic triad (His-362, Asp-411, Ser-507), which is directly responsible for the cleavage of C3b and C4b. Its structural features include:
-
Catalytically active site: induces a conformational change through substrate binding to release catalytic activity.
-
Disulfide bond linkage: forms a stable heterodimer with the heavy chain through disulfide bonds to prevent spontaneous activation.
Table 1 Structural and functional of CFI in the complement system.
|
Domain
|
Function
|
|
Heavy Chain
|
Substrate recognition, cofactor binding, structural stability
|
|
Light Chain
|
Catalytic activity for C3b/C4b cleavage
|
Biosynthesis and Expression
CFI is primarily synthesized in the liver, but can also be expressed by monocytes, fibroblasts and endothelial cells. The process of its synthesis includes the regulation of CFI activity that is tightly controlled by:
-
Precursor processing: generation of mature heterodimers by furin protease cleavage during translocation.
-
Glycosylation modification: both heavy and light chains contain N-linked glycosylation sites to enhance molecular stability and solubility.
Function of Complement Factor I
CFI serves as a central regulator of the complement system, ensuring precise control over immune activation while protecting host tissues from collateral damage. Its primary function revolves around cleaving activated complement components to terminate downstream signaling and prevent excessive inflammation.
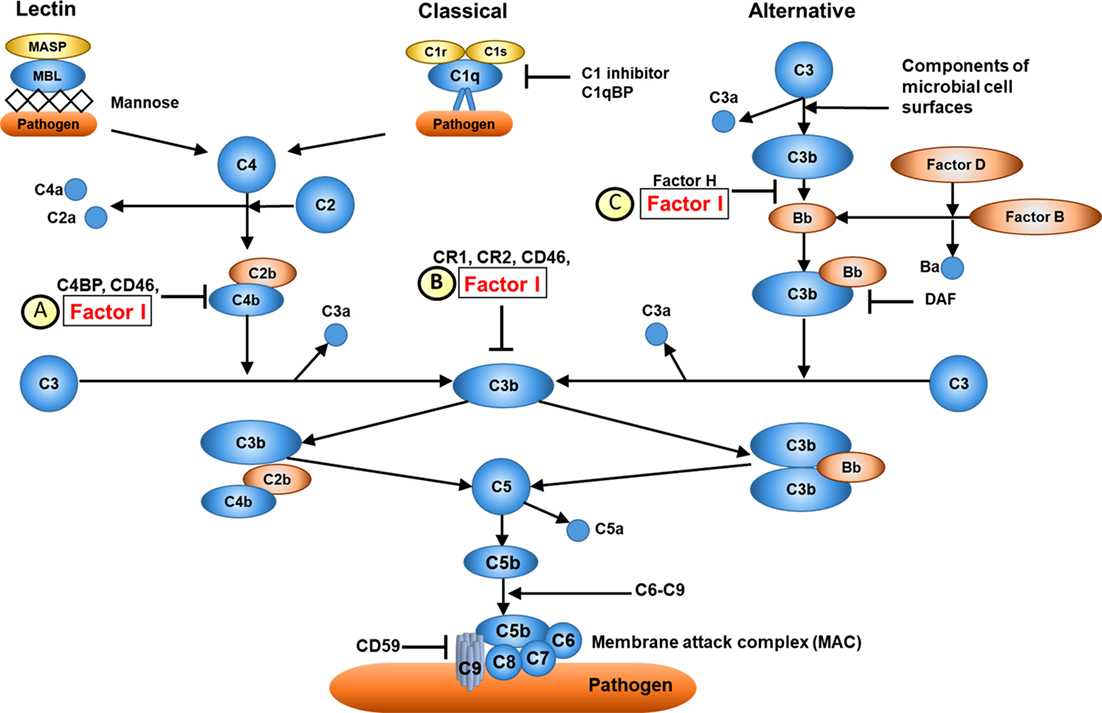 Fig. 1 Overview of complement factor I function.1,2
Fig. 1 Overview of complement factor I function.1,2
Primary Function: C3b/C4b Inactivation
CFI's core activity is proteolytic cleavage of C3b and C4b, the key components of C3/C5 convertases in the classical, lectin, and alternative pathways. By inactivating these substrates, CFI:
-
Prevents convertase formation: C3b and C4b are essential for assembling C3/C5 convertases, which amplify complement activation. Their cleavage halts this amplification loop.
-
Generates inactive fragments
-
C3b → iC3b → C3c/C3dg: Retains opsonization capacity but loses convertase activity.
-
C4b → C4d: A marker for immune complexes.
Cofactor Dependency
CFI's enzymatic activity is strictly cofactor-dependent, ensuring specificity for pathogen-bound substrates over host cells:
-
Cofactor binding: Proteins like Factor H, C4b-binding protein (C4BP), membrane cofactor protein (MCP/CD46), and CR1 bind to C3b/C4b, creating a platform for CFI-mediated cleavage.
-
Allosteric activation: Cofactor-substrate complexes induce structural changes in CFI, releasing its catalytic light chain from heavy-chain inhibition.
Regulation Mechanisms
CFI's activity is tightly controlled through:
-
Zymogen-like circulation: CFI exists in an inactive state in plasma, activated only upon cofactor-substrate binding.
-
Spatial restriction: Cleavage occurs exclusively on surfaces bound by cofactors (e.g., pathogens or immune complexes), sparing host cells.
-
Synergy with decay-accelerating factors: Proteins like DAF and C4BP accelerate convertase dissociation, complementing CFI's inactivation.
CFI functional assays are critical tools for evaluating the activity of this key regulator in the complement system. These assays assess CFI's ability to cleave C3b/C4b in the presence of cofactors, ensuring proper immune regulation and preventing pathological activation.
Key Assay Methods
Table 2 Methods of complement factor I testing.
|
Methods
|
Mechanism
|
Advantages
|
|
ELISA-based platforms
|
ELISA technology measures C3b/C4b cleavage efficiency by detecting iC3b or C4d fragments.
|
-
Standardized
-
Rapid (results in ~3 hours)
-
Adaptable to automation
|
|
SDS-PAGE analysis
|
Use radiolabeled or fluorescently tagged C3b/C4b to visualize cleavage products (iC3b, C3dg, C4d) via gel electrophoresis.
|
-
Directly assesses proteolytic activity
-
Distinguishes between partial/complete functional impairment
|
Assay Design Considerations
-
Cofactor dependency: Assays require cofactors (Factor H, CR1, C4BP) to mimic physiological conditions.
-
Standardization: ELISA platforms ensure reproducibility, while SDS-PAGE/Luminex provide mechanistic insights.
-
Combination strategies: Integrating multiple assays (e.g., SDS-PAGE + ELISA) improves sensitivity for detecting subtle functional deficits.
Factor I and Disease Pathology
CFI is a key regulator of the complement system, primarily responsible for cleaving C3b and C4b to prevent uncontrolled complement activation. Dysregulation of CFI due to mutations or functional defects can lead to a range of disorders, from immunodeficiency to autoimmune and inflammatory diseases.
CFI deficiency impairs the ability of the complement system to regulate C3b/C4b, leading to long-term C3 depletion and reduced conditioning. This can lead to:
-
Recurrent bacterial infections (e.g. Neisseria spp., Streptococcus pneumoniae) - due to impaired pathogen clearance.
-
Life-threatening complications - such as pneumonia, meningitis and sepsis.
-
Low serum C3 levels - this is a marker of CFI deficiency and exacerbates immune dysfunction.
Uncontrolled complement activation in CFI deficiency triggers an autoimmune response:
-
Systemic Lupus Erythematosus (SLE) - Impaired clearance of immune complexes and chronic inflammation can lead to SLE-like symptoms.
-
Rheumatoid arthritis - Complement dysregulation leads to joint inflammation and tissue damage.
CFI mutations are strongly associated with complement-mediated renal disease:
-
Atypical hemolytic uremic syndrome (aHUS)-Mutations in CFI or cofactors (e.g., factor H) impair C3b regulation, leading to endothelial injury and thrombotic microangiopathy.
-
C3 glomerulopathy (C3G)-Partial deficiency of CFI leads to dysregulation of alternative pathway activity, resulting in C3 deposition in the glomerulus.
CFI deficiency can manifest as isolated CNS inflammation.
CFI mutations cause retinal inflammation and progression of AMD.
CFI deficiency exemplifies the "double-edged sword" nature of the complement system, with dysregulation leading to immunodeficiency and tissue damage. Its role in central nervous system inflammation, kidney disease, and AMD underscores the need for early diagnosis and targeted therapy. Functional tests and advances in complement inhibitors hold promise for the treatment of these complex diseases.
Factor I as a Therapeutic Target
CFI plays a role in regulating complement activation and preventing immune-mediated tissue damage. Its function in degrading C3b and C4b prevents excessive complement activity, reducing inflammation and tissue damage. Dysregulation of CFI is linked to autoimmune diseases, inflammatory disorders, and complement-mediated pathologies, making it an attractive therapeutic target.
The following are key therapeutic strategies for CFI-related conditions that are supported by clinical evidence and emerging research.
Table 3 Therapeutic strategies targeting factor I.
|
Approach
|
Mechanism
|
Potential Applications
|
|
Factor I replacement
|
Administer recombinant Factor I to restore complement regulation
|
aHUS, C3G
|
|
Factor I activation enhancement
|
Small molecules or biologics that increase factor I activity
|
Autoimmune diseases, inflammatory disorders
|
|
Inhibition of factor I
|
Monoclonal antibodies or inhibitors to reduce excessive factor I activity
|
AMD, immunodeficiency disorders
|
|
Gene therapy
|
Corrects genetic defects in Factor I production
|
Rare complement deficiencies
|
In addition to this, the role of CFIs in immunomodulation puts them in the spotlight in the following areas:
-
Biomarker discovery: CFI levels correlate with complement activation in autoimmune and infectious diseases.
-
Drug development: Small molecule modulators of CFI-cofactor interactions are under investigation.
-
Gene therapy: Correction of CFI mutations in deficiencies.
CFI is key to immune regulation, balancing pathogen clearance with host protection. As research progresses, the therapeutic potential of CFIs offers promising avenues for the treatment of immune-related diseases. For Creative Biolabs, this knowledge underscores the importance of targeting CFIs in the drug discovery process, ensuring tailored solutions for complement-mediated diseases.
Creative Biolabs offers a full range of complement-related services and products, including:
If you want more information, please feel free to contact us.
References
-
Nanthapisal, Sira, et al. "Cutaneous vasculitis and recurrent infection caused by deficiency in complement factor I." Frontiers in Immunology 9 (2018): 735. https://doi.org/10.3389/fimmu.2018.00735
-
under Open Access license CC BY 4.0, without modification.
For Research Use Only.
Related Sections:

 Fig. 1 Overview of complement factor I function.1,2
Fig. 1 Overview of complement factor I function.1,2