CFB Structure CFB Functions CFB Test CFB Deficiency Therapeutic Target
Among the three activation pathways of the complement system, the alternative pathway stands out for its unique ability to amplify the immune response without antigen-specific recognition. Central to this amplification is complement factor B (CFB), a multifunctional protein that orchestrates key enzymatic steps in the cascade.
Table 1 Key steps in the complement alternative pathway.
|
Step
|
Molecular Event
|
Function
|
|
C3 hydrolysis
|
Spontaneous hydrolysis of C3 to C3a and C3b
|
Alternative pathway initiation point
|
|
CFB binding
|
C3b binds to CFB
|
Formation of the C3bB complex
|
|
Factor D cleavage
|
Factor D cleaves CFB to form Ba and Bb
|
Activation of C3 convertase
|
|
C3 cleavage
|
C3bBb complex cleaves C3
|
Amplifies complement activation
|
|
C5 convertase formation
|
C3bBb complex binds a second C3b
|
Converts to C5 convertase
|
Structure and Properties of Complement Factor B
Complement Factor B, also known as CFB, is a single-chain, 93-kDa polypeptide. It is part of the alternate pathway of the complement system and circulates in the blood as a single chain polypeptide. CFB exists in human serum in an inactive zymogen conformation, which itself consists of a three-complement control protein (CCP) domain, a von Willebrand factor type A (vWFA) module, and a serine protease domain (Fig.1). After binding to C3b, CFB is recognized by factor D and hydrolyzed between its CCP and vWFA domains by factor D, leading to the formation of the heterodimer complex C3bBb, the C3 convertase of the complement alternative pathway.
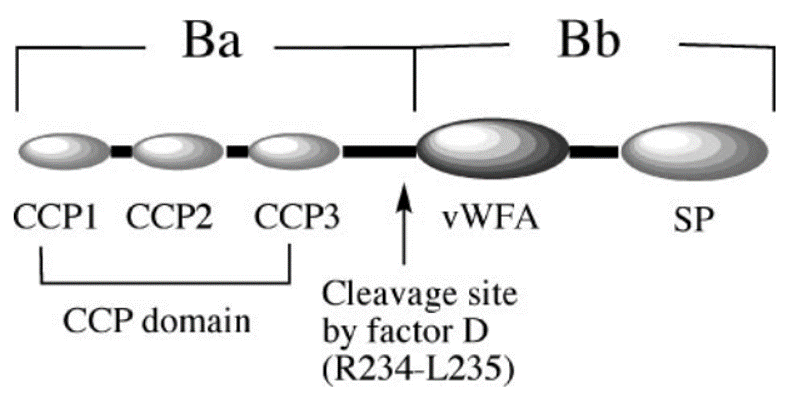
Fig. 1 Schematic representation of factor B showing components CCP1–3 (three-complement control proteins), vWFA (von Willebrand factor type A), and SP (serine protease).1,3
CFB is encoded by the CFB gene located in the MHC class III region on chromosome 6. It circulates in plasma as a zymogen (inactive precursor) until activated by the alternative pathway. Upon activation, CFB undergoes proteolytic cleavage by factor D, yielding two fragments:
-
Ba: A noncatalytic fragment (~33 kDa) containing three short consensus repeat (SCR) domains critical for initial binding to C3b.
-
Bb: A catalytic subunit (~60 kDa) with serine protease activity, responsible for forming the C3/C5 convertase complex.
The structural organization of CFB ensures strict regulation. The binding of C3b to CFB induces a conformational rearrangement, displacing inhibitory helices and exposing the scissile bond for cleavage by factor D. This allosteric mechanism ensures that CFB remains dormant until recruited to pathogen surfaces.
Functions of Complement Factor B
CFB plays a crucial role in the complement system, especially during the activation of the alternative pathway. Its primary function is to participate in the immune response in cooperation with other complement components to remove pathogens and damaged cells.
Role in the Alternative Complement Cascade
The alternative pathway is unique in its ability to amplify complement activation through a feedback loop. CFB is pivotal in two key steps:
-
Formation of the C3 Convertase
After cleavage by factor D, the Bb fragment remains covalently bound to C3b, forming the C3bBb complex (C3 convertase). This enzyme cleaves C3 into C3a (anaphylatoxin) and C3b, which deposits on microbial surfaces to recruit additional CFB and factor D, perpetuating the cycle.
-
Transition to the C5 Convertase
The C3 convertase undergoes a substrate shift upon binding to a second C3b molecule, forming the C3bBbC3b complex (C5 convertase). This enzyme cleaves C5 into C5a (a potent chemotactic factor) and C5b, initiating terminal pathway activation and membrane attack complex (MAC) formation.
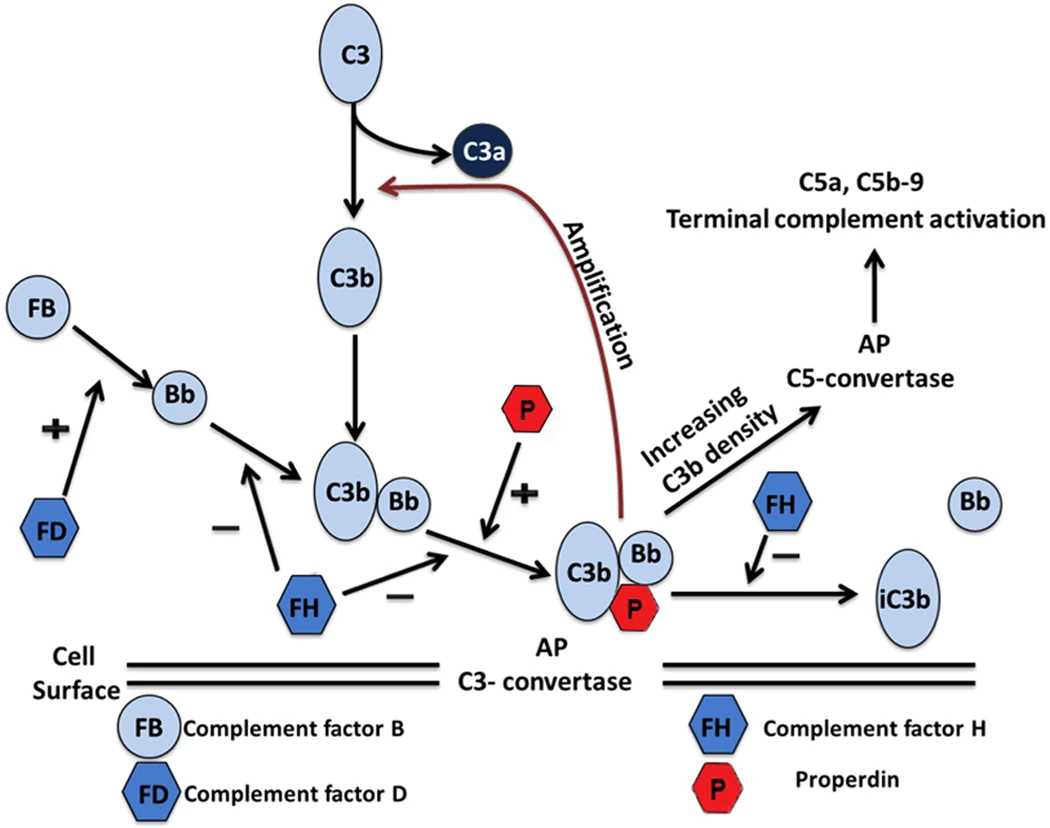 Fig. 2 Activation of the alternative complement pathway.2,3
Fig. 2 Activation of the alternative complement pathway.2,3
Regulation of the Inflammatory Response
The role of CFB is not limited to complement activation. It also plays an important role in the regulation of inflammatory processes.
-
During the immune response, activation of CFB promotes the release of inflammatory mediators, which recruit immune cells such as neutrophils and macrophages to the site of infection. The recruitment and activation of such cells help to enhance the local immune response and improve the clearance efficiency of pathogens.
-
Meanwhile, since activation of CFB leads to the production of large amounts of C3b, which labels pathogens and facilitates their recognition and clearance by phagocytes, it connects the bridge between the complement system and cellular immunity.
Other Biological Functions
In addition to its role in complement activation and inflammatory response, CFB has other biological functions.
-
Studies have shown that CFB may be involved in regulating the activation and proliferation of T cells, which play an important role in the clearance of viruses and tumor cells, and the involvement of CFB may help to enhance the function of these cells.
-
In some cases, CFB has also been found to be involved in promoting B cell activation and antibody production, thus playing a role in adaptive immune responses.
Thus, CFB is not only a key component of the complement system, but also an important factor in regulating the overall immune response.
CFB is an important component of the alternative complement pathway and plays a central role in immune defense and disease pathogenesis. CFB testing assesses its functional activity or antigenic levels, providing insight into complement system regulation and associated diseases.
Table 2 Test methods for complement factor B.
|
Approaches
|
Principle
|
|
ELISA
|
A widely used method where CFB is captured by a pre-coated antibody, followed by detection with a secondary antibody conjugated to an enzyme (e.g., horseradish peroxidase). This method quantifies CFB levels in serum or plasma.
|
|
Hemolytic Assays
|
Measures the functional integrity of the alternative pathway by assessing CFB’s role in forming the C3 convertase (C3bBb).
|
|
Nephelometry
|
Measures CFB protein levels using light-scattering immune complexes. This method is less common but provides direct antigenic quantification.
|
|
Flow Cytometry
|
Assesses CFB binding to C3b-coated surfaces, reflecting its role in convertase formation.
|
|
SPR
|
Evaluates real-time binding kinetics between CFB and C3b.
|
Serum or plasma is the preferred sample for CFB testing. Abnormalities in their test results have been associated with:
Factor B Deficiency and Related Diseases
CFB deficiency is a rare genetic disorder that impairs the alternative complement pathway, leading to increased susceptibility to infection and potential autoimmune or inflammatory complications. CFB deficiency is caused by mutations in the CFB gene that disrupt the ability of the alternative pathway to form the enzyme C3 convertase (C3bBb).
CFB deficiency may exhibit a number of clinical manifestations.
-
Recurrent infections: Increased susceptibility to Neisseria meningitidis (meningococcal infections) and Neisseria gonorrhoeae, and susceptibility to infections such as pneumococcal pneumonia or sepsis.
-
Autoimmune and inflammatory diseases: Dysregulation of complement activation may lead to autoimmune tissue damage, as in SLE.
-
C3 glomerulopathy (C3G): Autoantibodies that stabilize the C3 converting enzyme (e.g., anti-factor B autoantibodies) can lead to kidney damage.
-
Infectious shock: Impaired bacterial clearance exacerbates systemic inflammation.
-
Alzheimer's disease and multiple sclerosis: Chronic complement activation may lead to neuroinflammation.
Table 3 Diagnostic features.
|
Test
|
Findings
|
Clinical Significance
|
|
AH50 Test
|
Low alternative pathway activity
|
Confirmation of CFB deficiency
|
|
CFB antigen level
|
Very low (<1 mg/dL)
|
Direct evidence of deficiency
|
|
C3 level
|
Moderately low
|
Reflects chronic complement depletion
|
|
Genetic testing
|
CFB gene mutation
|
Confirmation of genetic basis
|
CFB plays a central role in the alternative pathway, making it a versatile target for the treatment of complement-driven diseases. Ongoing research is aimed at improving specificity, improving delivery, and expanding its use in diseases such as AMD and RA.
Table 4 Disease-specific applications.
|
Disease
|
Therapeutic Approach
|
Key Outcomes
|
|
PNH
|
Iptacopan
|
Reduced hemolysis, improved hemoglobin levels
|
|
IgAN
|
Iptacopan
|
Accelerated approval for proteinuria reduction
|
|
C3G
|
Iptacopan
|
Potential to inhibit complement-driven kidney damage
|
|
RA
|
siRNA
|
Reduced synovial inflammation in preclinical models
|
|
AMD
|
Iptacopan
|
Targeting complement-mediated retinal damage
|
CFB is central to the alternative complement pathway and has emerged as a promising therapeutic target for the treatment of complement-mediated diseases. The therapeutic strategies against CFB that are currently under investigation are:
Small molecule inhibitors
-
Mechanism: Binds to the active site of the Bb subunit of CFB and inhibits the formation of the C3/C5 convertase.
-
Ongoing trials: Studies in C3G, aHUS and AMD.
siRNA therapy
-
Mechanism: siRNA inhibits hepatic CFB expression, thereby reducing systemic complement activation.
-
Optimization: Reduce sample processing time and risk of isolated activation by using instant detection technology.
Antibody-based approaches
There are a number of challenges associated with the current therapy, including:
-
Challenges in the mode of administration due to the large size of the antibody.
-
Higher doses are required to maintain inhibition due to higher plasma concentrations of CFBs.
CFB plays multifaceted functions in the immune system, ranging from promoting complement activation to modulating inflammation and influencing the activity of other immune cells. Understanding the mechanism of action of CFB not only helps to deepen the understanding of the complement system, but also provides potential targets for the development of new immunotherapeutic strategies.
Creative Biolabs offers a full range of complement-related services and products, including:
If you want more information, please feel free to contact us.
References
-
Le, Giang Thanh, Giovanni Abbenante, and David P. Fairlie. "Profiling the enzymatic properties and inhibition of human complement factor B." Journal of Biological Chemistry 282.48 (2007): 34809-34816. https://doi.org/10.1074/jbc.M705646200
-
Shahini, Negar, et al. "The alternative complement pathway is dysregulated in patients with chronic heart failure." Scientific reports 7.1 (2017): 42532. https://doi.org/10.1038/srep42532
-
under Open Access license CC BY 4.0, without modification.
For Research Use Only.
Related Sections:


 Fig. 2 Activation of the alternative complement pathway.2,3
Fig. 2 Activation of the alternative complement pathway.2,3