CFD Structure CFD Functions CFD Test CFD Deficiency Therapeutic Target
Complement component factor D (CFD), also known as adipsin, serves as a critical regulatory enzyme in the alternative pathway of the complement system. As the rate-limiting serine protease, CFD governs immune defense mechanisms while maintaining a delicate balance between physiological protection and pathological inflammation.
Factor D is uniquely involved in the alternative pathway, which provides continuous low-level immune surveillance and rapid amplification upon microbial detection. Unlike many other complement proteins synthesized as inactive precursors, CFD circulates in an active form, ready to participate in immune defense.
Structure of Complement Factor D
CFD is a serine protease of about 24 kDa consisting of a single polypeptide of 228 amino acids. Unlike other serine proteases in the complement system, CFD circulates in the plasma as a mature but 'resting-state' form at a very low concentration and is synthesized mainly in adipocytes and macrophages. The human CFD shares 98%, 96%, 84%, and 66% amino acid sequence homology with the chimpanzee, rhesus monkey, porcine, and mouse protein, respectively.
Its structure reveals a classical trypsin-like fold, but with a conformation uniquely stabilized by a disulfide-rich environment and minimal glycosylation.
Key features:
Table 1 Structure features of CFD.
|
Property
|
Description
|
|
Molecular Weight
|
~24 kDa
|
|
Structure
|
Serine protease (chymotrypsin-like)
|
|
Circulating Form
|
Constitutively active
|
|
Expression Site
|
Primarily adipose tissue and liver
|
|
Substrate Specificity
|
Cleaves factor B only in C3b-bound form
|
Function of Complement Factor D
CFD serves as the critical enzymatic initiator of the alternative pathway of complement activation, acting with remarkable specificity and precision. The primary function of factor D is to cleave factor B when it is bound to C3b, generating C3bBb, the C3 convertase of the alternative pathway. Unlike zymogens that require proteolytic activation, factor D circulates in a catalytically active form but is functionally inert until it encounters its sole natural substrate complex, factor B bound to C3b.
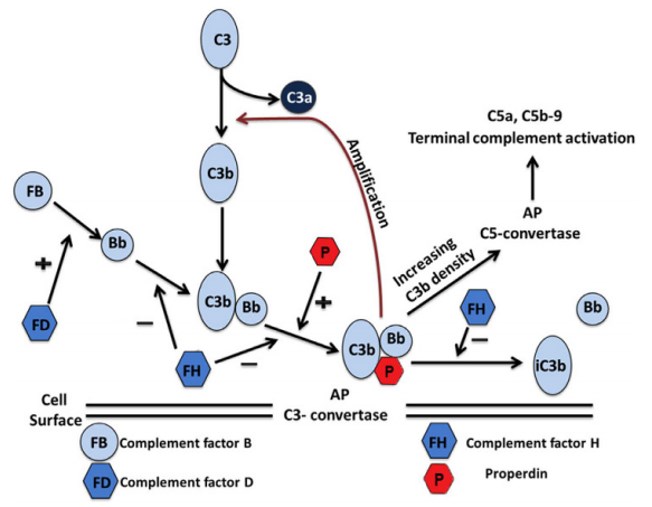 Fig. 1 Step-by-step mechanism of action of CFD.1,2
Fig. 1 Step-by-step mechanism of action of CFD.1,2
-
C3 spontaneous hydrolysis - Low-level spontaneous hydrolysis of native C3 generates C3(H2O), which initiates the alternative pathway in a continuous "tick-over" manner.
-
C3b deposition on target surfaces: C3(H2O) enables the formation of small amounts of C3b. If a pathogen or damaged cell surface is nearby, C3b binds covalently via a thioester bond.
-
Formation of pro-convertase (C3bB) - Soluble factor B binds to surface-deposited C3b, forming the pro-convertase complex (C3bB).
-
Factor D cleavage of factor B - Factor D cleaves factor B in the C3bB complex at a single peptide bond, releasing the Ba fragment and leaving Bb bound to C3b. This results in C3bBb (C3 convertase).
-
Amplification loop - The newly formed C3 convertase cleaves additional C3 into C3a and more C3b, exponentially increasing the amount of opsonin-decorated target surfaces. The cycle repeats as more C3b fragments deposit and engage further with factor B and factor D.
The precision and rate-limiting function of factor D make it irreplaceable in this cascade. Without it, the AP fails to assemble functional convertases, making it a bottleneck enzyme—and thus an attractive target for selective inhibition.
Accurate measurement and functional assessment of CFD are essential for both basic research and drug development programs targeting the alternative pathway. At Creative Biolabs, we offer a suite of high-precision assays tailored to quantify factor D expression, evaluate its enzymatic activity, and support inhibitor screening workflows.
Complement Factor D ELISA
We recommended approach is to employ the ELISA to achieve a concise functional delineation of CFD within serum or tissue specimens, as the role played by CFD is of paramount significance and singular distinction, as it orchestrates the cleavage of activated complement factor B into fragments Bb and Ba. Through this method, the operational dynamics of CFD can be effectively deduced by analyzing the resultant ELISA curve.
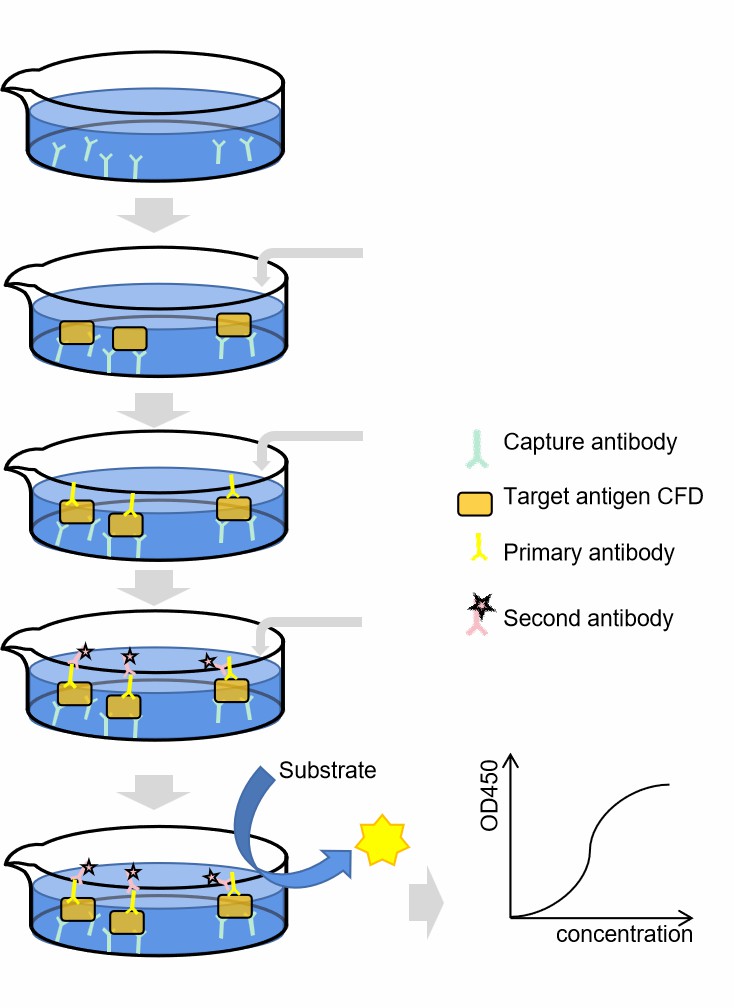 Fig. 2 Complement CFD ELISA workflow.
Fig. 2 Complement CFD ELISA workflow.
Hemolysis Test
We provide a tailored hemolysis assay involving the deployment of CFD-depleted serum (CFD-Dpl serum), which stands as a suitable method to functionally authenticate mutants of CFD.
The conventional hemolysis assay, employing rabbit erythrocytes, traditionally serves to unveil the impact of mutations on CFD. In this context, equivalent quantities of both CFD mutants and unaltered CFD are separately introduced into the CFD-Dpl serum, alongside rabbit erythrocytes. By gauging the extent of hemolysis, the ramifications of a mutation on CFD can be discerned. A comparative assessment can also be undertaken, appraising the quantum of CFD or its mutants requisite to restore 50% or 100% hemolysis within partially depleted CFD serum, thereby permitting the characterization of the mutational influence.
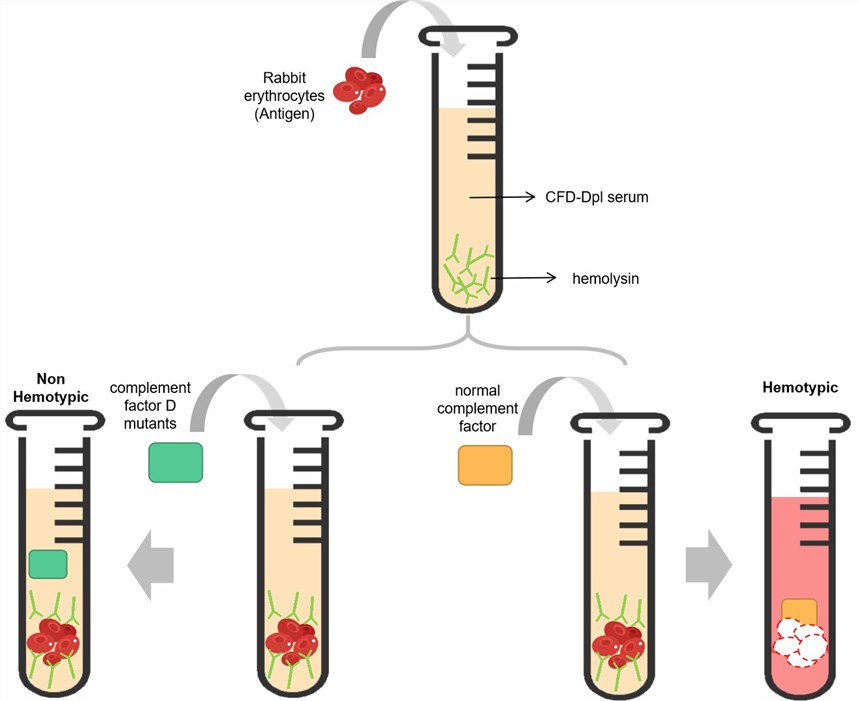 Fig. 3 Workflow of hemolysis test of CFD.
Fig. 3 Workflow of hemolysis test of CFD.
Factor D Deficiency and Related Diseases
Factor D levels are stable in healthy individuals and contribute to microbial defense, immune complex clearance, and apoptotic cell removal. Aberrant factor D activity or expression is implicated in several diseases.
Table 2 Pathological conditions of aberrant factor D.
|
Disease/Condition
|
Role of Factor D
|
|
Age-Related Macular Degeneration
|
Overactivation leads to retinal inflammation
|
|
Geographic atrophy (GA)
|
CFD-mediated C3 convertase drives retinal pigment epithelium degeneration
|
|
Atypical Hemolytic Uremic Syndrome (aHUS)
|
Uncontrolled alternative pathway activation
|
|
Paroxysmal Nocturnal Hemoglobinuria (PNH)
|
Amplifies C3 convertase activity on erythrocytes
|
|
C3 Glomerulopathy
|
Persistent C3 activation damages glomeruli
|
|
Obesity and Metabolic Syndrome
|
Elevated factor D expression from adipose tissue
|
These insights underscore the need for precise quantification and functional analysis of factor D, both in research and therapeutic development.
Factor D as a Therapeutic Target
Factor D is a prime candidate for selective complement inhibition, especially in diseases where alternative pathway dysregulation is dominant. Small-molecule inhibitors, monoclonal antibodies, and RNA-based approaches are under active development. Factor D possesses several characteristics that make it particularly attractive for drug development:
-
Low plasma concentration (~1–2 µg/mL), requiring minimal dosage for effective inhibition
-
Tight substrate specificity, limiting off-target effects
-
Rate-limiting enzymatic role, meaning its inhibition can completely shut down AP amplification
-
Constitutively active, removing the need for additional activation mechanisms
These features ensure that targeting factor D allows for precise, upstream control of alternative pathway activation. Several promising candidates are currently under clinical or preclinical evaluation. We offer a full suite of complement drug discovery services for drug discovery and comprehensive solutions for factor D investigation, including:
If you want more information, please feel free to contact us.
References
-
Shahini, Negar, et al. "The alternative complement pathway is dysregulated in patients with chronic heart failure." Scientific reports 7.1 (2017): 42532. https://doi.org/10.1038/srep42532
-
under Open Access license CC BY 4.0, without modification
For Research Use Only.
Related Sections:

 Fig. 1 Step-by-step mechanism of action of CFD.1,2
Fig. 1 Step-by-step mechanism of action of CFD.1,2
 Fig. 2 Complement CFD ELISA workflow.
Fig. 2 Complement CFD ELISA workflow.
 Fig. 3 Workflow of hemolysis test of CFD.
Fig. 3 Workflow of hemolysis test of CFD.