What is C3 C3 Pathway C3 Function C3 Deficiency C3 Test C3 Technologies
Among the complement proteins, complement component C3 stands out as a pivotal protein. Cleavage of C3 is a key event in all three complement activation pathways. The process is mediated primarily by enzyme complexes known as C3 convertases, which cleave C3 into two fragments, complement component C3a and C3b. This article will explore the structure, function, and significance of C3, including its various forms and its involvement in both normal and pathological immune responses.
What is C3 Complement?
Complement component C3 is a large, multi-functional protein that acts as a central player in the complement cascade. The complement system consists of a group of proteins that work together to enhance the body's immune defense mechanisms, particularly against infections. C3 is involved in various immune processes, such as opsonization, inflammation, and cell lysis, all of which are essential for pathogen elimination.
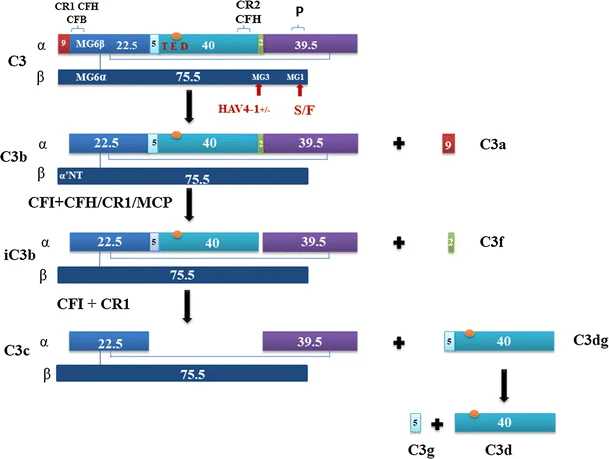 Fig. 1 The structure of the C3 protein and C3 proteolytic cascade.1,3
Fig. 1 The structure of the C3 protein and C3 proteolytic cascade.1,3
Complement C3 protein is synthesized as a single-chain precursor molecule that undergoes post-translational modification to form a two-chain structure. The mature protein consists of an α-chain (115 kDa) and a β-chain (75 kDa), linked by disulfide bonds, resulting in a total molecular weight of approximately 190 kDa. The protein's secondary structure comprises multiple domains, including the thioester-containing domain (TED), which is essential for its functional activity.
The C3 gene, located on chromosome 19, spans approximately 41 kb and contains 41 exons. Its expression is regulated by various inflammatory mediators and tissue-specific factors, ensuring appropriate complement system activity under different physiological conditions.
C3 is the most abundant complement protein in serum, with a concentration of about 1.2 mg/mL. Its activation is central to all three complement pathways: classical, alternative, and lectin.
C3 Pathway
C3 plays a key role in the complement cascade, particularly in the classical, alternative, and lectin pathways. The activation of C3 is a pivotal point in these pathways, leading to the generation of the active fragments C3a and C3b.
Complement C3c is a fragment of C3 generated during its cleavage. It is involved in complement activation and interacts with other complement proteins to promote immune responses. C3d is another important fragment that plays a role in enhancing the immune response, especially in antigen-presenting cells. C3d also contributes to the formation of immune complexes and helps improve the affinity of antibodies for their targets.
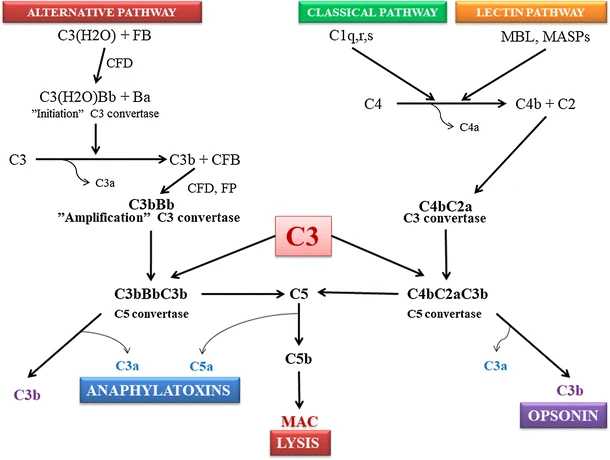 Fig. 2 The complement cascade.1,3
Fig. 2 The complement cascade.1,3
Table 1 C3 plays a key role in the complement cascade.
|
Complement Pathway
|
Description
|
|
Classical Pathway
|
This pathway is triggered by antibodies bound to antigens, which activate C1 and subsequently lead to the cleavage of C3 into C3a and C3b.
|
|
Alternative Pathway
|
This pathway is continuously active at low levels in the blood and can be spontaneously activated. It is amplified by microbial surfaces and does not require antibodies to initiate the cascade.
|
|
Lectin Pathway
|
Triggered by the binding of lectins (such as mannan-binding lectin) to specific carbohydrates on microbial surfaces, this pathway also leads to C3 activation.
|
All three pathways converge at C3, emphasizing its central role in complement activation. The formation of C3 convertase represents a critical step in this process.
C3 Convertase: Formation and Function
C3 convertase functions as the central enzymatic complex in the complement cascade. In the classical and lectin pathways, C4b2a serves as the C3 convertase, while in the alternative pathway, C3bBb performs this role. These convertases cleave C3 into its active fragments:
-
C3a: A potent anaphylatoxin
-
C3b: The major opsonin
-
C3c and C3d: Additional breakdown products
C3 convertase inhibitors play crucial roles in regulating this process, preventing excessive complement activation that could lead to tissue damage.
C3 Protein Function
The functions of C3 and its activation products are diverse and crucial for immune responses:
-
Opsonization: C3b acts as an opsonin, enhancing phagocytosis of targeted particles.
-
Immune Complex Clearance: C3b facilitates the removal of immune complexes from circulation.
-
Amplification: C3b participates in the formation of more C3 convertases, amplifying the complement response.
-
Inflammation: C3a acts as an anaphylatoxin, promoting inflammation.
-
B Cell Responses: C3d, a breakdown product of C3b, enhances B cell responses.
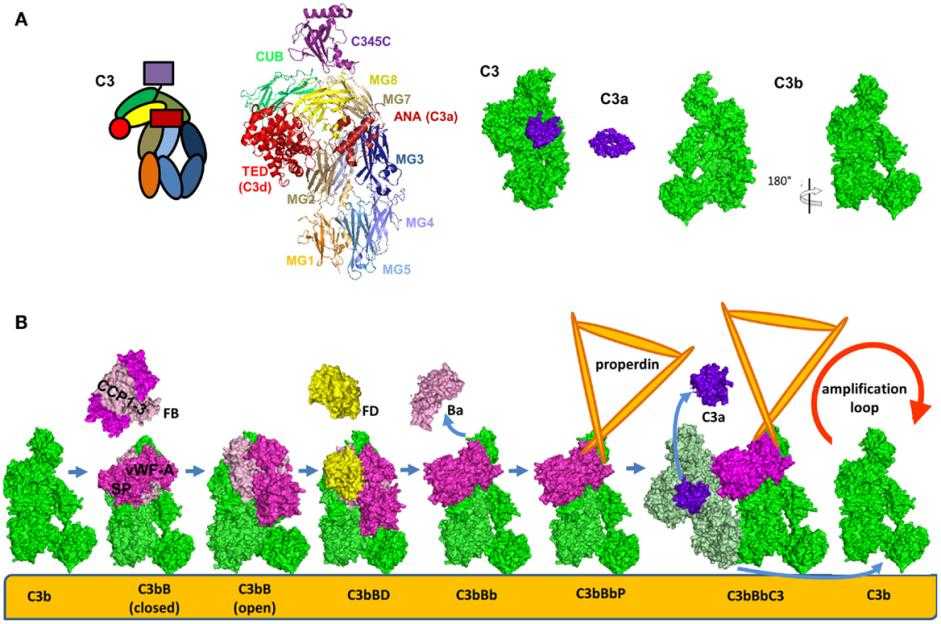 Fig. 3 Structure and domain organization of the central complement component C3 and its cleavage fragments C3b and C3a.2,3
Fig. 3 Structure and domain organization of the central complement component C3 and its cleavage fragments C3b and C3a.2,3
C3 Protein Deficiency
C3 deficiency is a rare condition that can lead to increased susceptibility to bacterial infections. Individuals with C3 deficiency may experience:
A C3 protein deficiency can lead to serious immune dysfunction. Individuals with low or absent C3 levels are at increased risk for recurrent bacterial infections, particularly from encapsulated organisms such as Streptococcus pneumoniae and Haemophilus influenzae. This is due to the impaired opsonization and lack of efficient immune clearance in the absence of functional C3.
Table 2 Clinical manifestations and role in disease models of C3 protein deficiency.
Clinical Manifestations
C3 protein deficiency, whether acquired or hereditary, can lead to severe clinical manifestations
|
Role in Disease Models
Research using mice models has significantly advanced our understanding of C3's role in various pathological conditions.
|
|
Recurrent bacterial infections
|
Mechanisms of complement regulation
|
|
Autoimmune disorders
|
Role in tissue homeostasis
|
|
Impaired immune complex clearance
|
Contribution to disease pathogenesis
|
|
Kidney disease
|
Potential therapeutic targets
|
Complement C3 Test
Testing for serum C3 levels can provide valuable information about the functioning of the complement system. Reduced C3 levels can indicate an ongoing immune response or an underlying disorder such as SLE or glomerulonephritis, where complement activation is dysregulated.
Serum C3 testing
Serum C3 testing serves as a valuable diagnostic tool in various clinical conditions. Normal C3 levels typically range from 90-180 mg/dL, though reference ranges may vary between laboratories. Variations in C3 levels can indicate different pathological conditions.
Table 3 Interpretation of C3 test results.
|
C3 Test Results
|
Possible Causes
|
|
High C3 levels
|
Acute inflammatory responses
|
|
Certain malignancies
|
|
Metabolic disorders
|
|
Low C3 levels
|
Autoimmune diseases
|
|
Hereditary C3 deficiency
|
|
Active SLE
|
It's important to note that C3 and C4 complement tests are often performed together to provide a more comprehensive assessment of complement activity.
C3 and C4 Complement Testing
Both C3 and C4 are essential components of the classical and lectin complement pathways. These proteins work together to mediate immune responses and pathogen clearance. A C3 and C4 complement test can help assess complement system activity and diagnose conditions such as autoimmune diseases or complement deficiencies.
Combined C3 and C4 testing provides valuable information. Several patterns may emerge.
-
C3 low, C4 normal: Often indicates alternative pathway activation
-
Both C3 and C4 low: Classical pathway activation
-
C3 complement high, C4 normal: May suggest acute phase response
C3 Technologies
The central role of C3 in complement activation makes it an attractive target for therapeutic interventions. C3 technologies are being developed to modulate complement activity in various diseases.
Table 4 Advanced C3 technologies
|
C3 Technologies
|
Function
|
|
C3 antibody development
|
These antibodies can block C3 activation, modulate complement activity and treat complement-mediated diseases.
|
|
C3 inhibitors
|
These compounds aim to reduce excessive complement activation in conditions like age-related macular degeneration.
|
|
C3-targeted therapies
|
Approaches to selectively inhibit or enhance C3 function in specific disease contexts.
|
Recent studies have shed light on novel aspects of C3 biology and its potential applications.
Structure-function relationships
Ongoing research into C8 to C3 secondary structure interactions continues to reveal new aspects of complement activation and regulation. These studies provide insights into:
-
Protein-protein interactions
-
Conformational changes
-
Regulatory mechanisms
-
Therapeutic targeting
Emerging therapeutic strategies
-
C3 convertase inhibitors
-
Targeted complement modulators
-
Cell-specific complement regulators
-
Bioengineered complement proteins
C3 biology
-
AI algorithms capable of generating novel protein structures, including those related to complement proteins like C3
-
The rapid generation and screening of large numbers of antibodies, facilitating the discovery of highly specific anti-C3 antibodies for research
-
Structure-guided engineering of C3-related proteins
Complement component C3 stands as a cornerstone of the innate immune system, playing diverse roles in host defense, inflammation, and immune regulation. Its complex structure and multifaceted functions make it a fascinating subject of study and a promising target for therapeutic interventions.
The ongoing research into C3 and related complement proteins continues to unveil new insights into immune system function and offers exciting prospects for treating a wide range of diseases associated with complement dysregulation. Creative Biolabs offers a full range of complement-related services and products, including:
If you want more information, please feel free to contact us.
References
-
Łukawska, Emilia, Magdalena Polcyn-Adamczak, and Zofia I. Niemir. "The role of the alternative pathway of complement activation in glomerular diseases." Clinical and experimental medicine 18 (2018): 297-318. https://doi.org/10.1007/s10238-018-0491-8
-
Merle, Nicolas S., et al. "Complement system part I–molecular mechanisms of activation and regulation." Frontiers in immunology 6 (2015): 262. https://doi.org/10.3389/fimmu.2015.00262
-
under Open Access license CC BY 4.0, without modification.
For Research Use Only.
Related Sections:

 Fig. 1 The structure of the C3 protein and C3 proteolytic cascade.1,3
Fig. 1 The structure of the C3 protein and C3 proteolytic cascade.1,3
 Fig. 2 The complement cascade.1,3
Fig. 2 The complement cascade.1,3
 Fig. 3 Structure and domain organization of the central complement component C3 and its cleavage fragments C3b and C3a.2,3
Fig. 3 Structure and domain organization of the central complement component C3 and its cleavage fragments C3b and C3a.2,3