What is C6? C6 Function C6 Test C6 Deficiency C6 Therapeutics
Complement component C6 plays a pivotal role in the innate immune system by contributing to the formation of the membrane attack complex (MAC), a cytolytic structure responsible for direct microbial killing. Creative Biolabs recognizes the importance of C6 not only as a fundamental immune protein but also as a target and tool for immunological and therapeutic research.
What is Complement C6?
C6 is a single-chain glycoprotein composed of 913 amino acids, encoded by a gene on chromosome 5p13. C6 belongs to the membrane attack complex/perforin (MACPF) superfamily. Upon C5b generation, C6 binds stably to form the C5b6 complex, initiating the nucleation of MAC on target membranes.
Structurally, C6 is modular and exhibits significant homology with other terminal complement components (TCCs) - notably C7, C8, and C9 - reflecting their shared evolutionary origins and functional interdependence. The C6 protein is composed of multiple domains, including:
-
MACPF domain - shares structural homology with pore-forming proteins like perforin
-
EGF-like domain - thought to mediate protein–protein interactions
-
Thrombospondin (TSP) repeats - involved in target binding
-
Low-density lipoprotein receptor class A (LDLa) domain
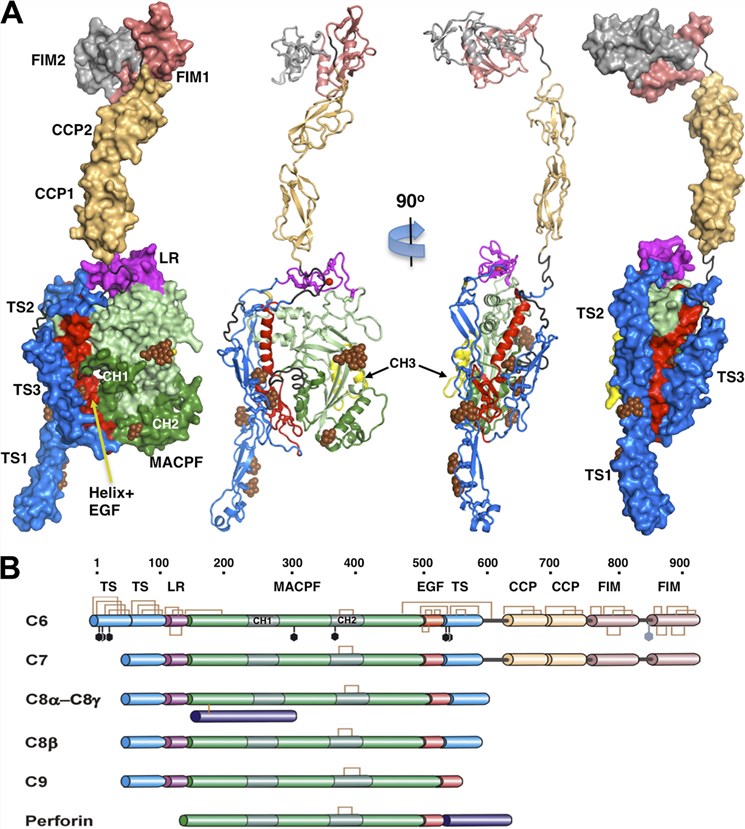 Fig. 1 Crystal structure and domain organization of C6.1, 2
Fig. 1 Crystal structure and domain organization of C6.1, 2
The crystal structure of C6 reveals a "closed," autoinhibited conformation in its resting state, which undergoes a dramatic conformational change upon activation, facilitating its integration into the growing MAC.
Functional Role of C6 in the Complement System
C6 plays a central role in the terminal phase of the complement cascade, which is a crucial part of the innate immune system’s defense against pathogens.
-
Initiation: C5 is cleaved by C5 convertase, generating C5b.
-
Assembly: C5b sequentially binds C6, C7, C8, and multiple C9 molecules to form the MAC.
-
Pore formation: The MAC inserts into target cell membranes, creating a transmembrane channel that leads to osmotic lysis of pathogens or infected cells.
Table 1 C6 in the complement cascade.
|
Step
|
Role of C6
|
|
C5 activation
|
C5 is cleaved to C5b
|
|
C5b binding
|
C5b binds to C6, forming the C5b-6 complex
|
|
MAC assembly
|
C5b-6 recruits C7, C8, and C9 to build the MAC
|
|
Effector function
|
MAC forms pores, causing target cell lysis
|
|
Inflammatory signaling
|
Sublytic MAC can trigger host cell inflammatory responses
|
In summary, C6 is a critical structural and functional component of the terminal complement pathway, essential for the assembly and activity of the MAC, and thus for effective immune defense and regulation of inflammation.
C6 Functional Test
Evaluating the functionality of C6 is essential for both basic immunological research and clinical investigations into complement-related disorders. At Creative Biolabs, we provide a comprehensive C6 functional assay platform designed to determine the biological activity of C6 within the terminal pathway of the complement cascade.
C6 functional testing assesses the ability of native or recombinant C6 to participate in MAC formation, specifically its interaction with C5b and subsequent recruitment of C7 - C9 components. This test is critical in:
-
Diagnosing C6 deficiency
-
Screening C6-targeting drug candidates
-
Evaluating complement activity in therapeutic monoclonal antibody development
-
Quality control for C6 protein production or purification
Table 2 Methodologies for C6 functional testing.
|
Assay Type
|
Principle
|
Application
|
|
Hemolytic CH50 Assay
|
Measures total terminal pathway activity via red blood cell lysis
|
Indirectly reflects C6 functionality in serum
|
|
C5b-6 Reconstitution Assay
|
Uses C6-depleted serum reconstituted with test C6 protein
|
Specific for evaluating recombinant or mutant C6 activity
|
|
ELISA-Based C5b-9 Formation
|
Detects C5b-9 complex formation on cell or artificial surfaces
|
Functional endpoint readout of complete MAC assembly
|
|
Surface Plasmon Resonance (SPR)
|
Quantifies C6 interaction with C5b and C7 in real-time
|
Mechanistic or inhibitor screening studies
|
C6 in Health and Disease
In healthy individuals, C6 promotes the terminal pathway of the complement system. C6 is able to directly lyse invading pathogens and plays a front-line role in innate immunity, especially against Neisseria. It also prevents the accumulation of immune complexes, thereby maintaining tissue integrity.
Primary C6 deficiency is a rare autosomal recessive disorder marked by absent or dysfunctional C6 protein. Excessive or misdirected activation of MAC, particularly via overactive C6, has been implicated in several chronic conditions.
-
Atypical hemolytic uremic syndrome (aHUS): MAC-induced endothelial injury
-
Age-related macular degeneration (AMD): C6 contributes to retinal cell damage
-
Systemic lupus erythematosus (SLE): Terminal complement activity can drive tissue destruction
In such diseases, therapeutic inhibition of C6 or MAC formation represents a promising approach to reduce collateral tissue damage while preserving upstream complement functions such as opsonization and chemotaxis.
Recent findings reveal novel roles for C6 in non-traditional domains:
Table 3 Emerging roles of C6 in neurology and oncology.
|
Diseases
|
Roles of C6
|
|
Neurodegenerative Disorders
|
-
Upregulated MAC components, including C6, have been detected in Alzheimer’s disease, where they may contribute to synaptic loss and neuronal apoptosis.
-
MAC disruption is now being explored as a neuroprotective strategy in amyotrophic lateral sclerosis (ALS) and Parkinson’s disease.
|
|
Cancer
|
-
Some tumors exploit complement dysregulation to evade immune detection.
-
C6 expression patterns are being investigated as biomarkers for immune status in tumor microenvironments.
-
C6-modulating therapies may enhance the efficacy of checkpoint inhibitors or oncolytic viruses.
|
Component C6-Based Therapy
Positioned at a critical juncture in the MAC assembly, C6 offers a unique opportunity for selective modulation of terminal complement activity, preserving early immune functions while minimizing tissue-damaging effects. Unlike upstream complement components (e.g., C3, C5), which are involved in multiple immune pathways, C6 specifically participates in MAC formation, downstream of immune signaling and opsonization. This makes it an attractive target in conditions where terminal lysis drives pathology but immunosurveillance must be preserved.
-
Neutralizing antibodies - Bind to C6 to block its interaction with C5b or prevent MAC assembly
-
Small molecule inhibitors - Designed to disrupt conformational activation of C6 or its recruitment to C5b
-
siRNA and ASO Therapies - Silence hepatic expression of C6 to reduce systemic MAC formation
-
Aptamers and peptide-based inhibitors - New modalities with high specificity and tunable half-life
C6 is more than just a MAC subunit - it's a critical immune mediator with broad implications for infection control, inflammation, and therapeutic targeting. From congenital deficiencies to emerging roles in cancer and neurodegeneration, C6 continues to be a focal point of complement biology. We offer a full suite of tools and services to support C6-targeted drug discovery.
If you want more information, please feel free to contact us.
References
-
Aleshin, Alexander E., et al. "Structure of Complement C6 Suggests a Mechanism for Initiation and Unidirectional, Sequential Assembly of Membrane Attack Complex (MAC)*♦." Journal of Biological Chemistry 287.13 (2012): 10210-10222. https://doi.org/10.1074/jbc.M111.327809
-
under Open Access license CC BY 4.0, without modification
For Research Use Only.
Related Sections:

 Fig. 1 Crystal structure and domain organization of C6.1, 2
Fig. 1 Crystal structure and domain organization of C6.1, 2