Alternative Pathway Basics Amplification and Regulation Effector Functions Experimental Approaches Resources
The alternative pathway is one of three complement pathways that lead to activation of the complement cascade. The alternative pathway does not require a specific antibody to begin, so it can be much faster and more efficient than in the case where antibody synthesis is required in the classical pathway. Instead, in the alternative pathway of complement activation, the C3 protein directly binds to microorganisms and only certain types of antigens can activate this pathway. The alternative pathway can be activated by bacteria, viruses, fungi, cobra venom, parasites, IgA immune complexes and polysaccharides and form an important part of the defense mechanism independent of the immune response.
Overview of the Alternative Pathway
The alternative pathway activation occurs on microbial surfaces in the absence of specific antibody. The alternative pathway is activated slowly by the spontaneous hydrolysis of the internal C3 thioester bond and further triggered by contact with various proteins, lipids and carbohydrate structures on microorganisms and other foreign surfaces. Activation of the alternative pathway triggers a cascade involving C3 and factors B and D and properdin, result in cleavage of C5 and formation of the membrane attack complex (MAC), which in its final state creates a pore in the cell wall and causes cell lysis (Fig.1).
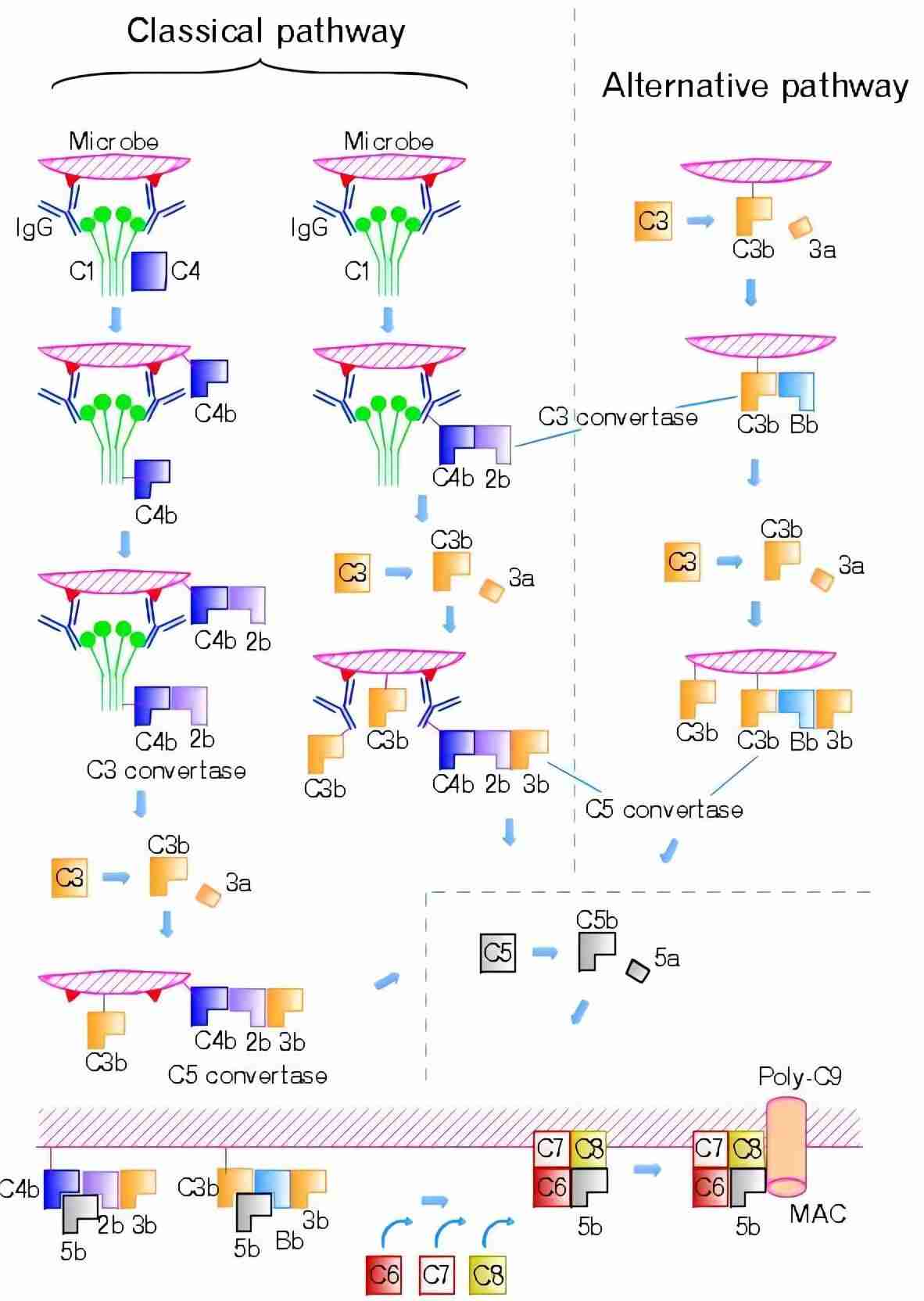
Fig. 1 Alternative and classic complement pathways.1
Activation of the Alternative Pathway
The alternative pathway of complement activation is initiated by the spontaneous hydrolysis of complement component C3, resulting in the formation of C3(H2O). This C3(H2O) molecule is then capable of interacting with factor B, which, upon cleavage by factor D, forms the C3 convertase complex (C3bBb). The C3 convertase plays a critical role in the amplification of the complement cascade, cleaving additional C3 molecules into C3b and C3a.
The process can be broken down into several key steps:
Table 1 Several key steps of the alternative pathway.
|
Key Steps
|
Description
|
|
Spontaneous Hydrolysis of C3
|
Under normal physiological conditions, C3 undergoes spontaneous hydrolysis, resulting in C3(H2O). This is the starting point of the alternative complement pathway.
|
|
Formation of C3 Convertase
|
The binding of C3(H2O) to factor B leads to the cleavage of factor B by factor D, forming the C3 convertase, C3bBb.
|
|
Amplification Loop
|
The C3 convertase cleaves additional C3 molecules, generating C3b. C3b then binds to pathogen surfaces, marking them for opsonization or further activation of the complement cascade.
|
|
Formation of C5 Convertase
|
The C3bBb complex, in the presence of additional C3b molecules, forms the C5 convertase (C3bBbC3b), which cleaves C5 into C5a and C5b. The latter initiates the terminal complement cascade, leading to the formation of the MAC.
|
|
MAC
|
The MAC, formed by the sequential binding of C5b, C6, C7, C8, and C9, creates a pore in the target cell membrane, leading to cell lysis.
|
Key Components of the Alternative Pathway
Several critical proteins contribute to the activation and regulation of the alternative pathway complement.
-
C3: The central protein in all complement pathways, C3 is cleaved into C3a and C3b, the latter of which is involved in opsonization and the formation of C3 convertase.
-
Factor B: A complement protein that binds to C3(H2O) or C3b to form the C3 convertase (C3bBb) upon cleavage by factor D.
-
Factor D: An enzyme that cleaves factor B when bound to C3(H2O) or C3b, facilitating the formation of the C3 convertase.
-
Properdin: A stabilizing factor that binds to the C3bBb complex, enhancing the stability and activity of the C3 convertase.
-
Factor H: A regulatory protein that competes with factor B for binding to C3b and promotes the dissociation of C3bBb. Factor H also acts as a cofactor for factor I, which cleaves C3b to form inactive iC3b, preventing excessive complement activation.
-
Factor I: An enzyme that cleaves C3b into inactive fragments (iC3b), helping to regulate complement activation and prevent host tissue damage.
-
CD55 (Decay-Accelerating Factor): A membrane-bound protein that accelerates the decay of C3 convertase complexes, preventing prolonged complement activation.
-
CD46 (Membrane Cofactor Protein): Another regulatory protein that serves as a cofactor for factor I to cleave C3b and limit the activation of the complement system.
Amplification and Regulation of the Alternative Pathway
The key amplification step in the alternative pathway involves the generation of C3 convertases, which cleave C3 into C3a and C3b. The C3b fragments can initiate the formation of larger complexes, including the C5 convertase, leading to the downstream activation of the terminal complement pathway.
There are two primary forms of C3 convertases in the alternative pathway:
-
C3bBb: This is the fluid-phase C3 convertase formed during the tick-over process and is the primary activator in the amplification loop.
-
C3bBbP: The properdin-stabilized C3 convertase, which is more stable and has a higher affinity for surface-bound C3b, enhancing its activity.
The conversion of C3 to C3b leads to further amplification of the complement cascade, resulting in increased pathogen opsonization, recruitment of inflammatory cells, and the eventual formation of the MAC that can lyse pathogen membranes.
Regulation of Alternative Pathway
To prevent the complement cascade being activated against the body's own cells, there are several different kinds of regulatory proteins that disrupt the complement activation process. These include serum-based and cell-based factors that inactivate C3b when it is produced. Serum-based factors include complement factor H and factor I. The cell-based receptors include CD46 or membrane cofactor protein (MCP) and thrombomodulin. Mutations or loss of all of these regulatory molecules can cause uncontrolled activation of the alternative pathway, which can lead to diseases such as atypical hemolytic uremic syndrome (aHUS).
Each regulatory protein can promote C3b inactivation and prevent further progression of the complement cascade.
-
Factor H binds C3b and works with factor I to inactivate it.
-
MCP can also bind to C3b which has become attached to cells and works with factor l to inactivate it.
-
Thrombomodulin regulates complement by inactivating the pro-inflammatory mediators C3a and C5a and accelerating factor l-mediated C3b inactivation because mutations are associated with aHUS. Thrombomodulin also plays a role in the regulation of local coagulation through interaction with thrombin.
Inhibitory Mechanisms
The alternative pathway's regulation also involves controlling the stability and activity of the C3 convertases. Properdin, a positive regulator, stabilizes the C3bBb complex on pathogen surfaces, enhancing the complement response. In contrast, the regulatory proteins mentioned above provide inhibitory mechanisms to dampen the response on host cells or in the fluid phase.
These inhibitory processes are vital for maintaining the delicate balance between activating the immune system when necessary and preventing damage to self tissues, which could lead to inflammatory diseases, including autoimmune disorders such as systemic lupus erythematosus (SLE) and age-related macular degeneration (AMD).
Effector Functions of the Alternative Pathway
The alternative pathway plays a critical role in early immune responses. This pathway operates in a constantly active, low-level state, but can be rapidly amplified when needed. The effector functions of the alternative pathway are pivotal for eliminating pathogens and modulating immune responses.
Table 2 Effector functions of the alternative pathway.
|
Functions
|
Descriptions
|
Mechanisms
|
|
Opsonization and Phagocytosis
|
When C3b binds to microbial surfaces, it marks the pathogen for recognition and ingestion by phagocytes, such as macrophages and neutrophils. This process enhances the efficiency of phagocytosis, as complement receptors (e.g., CR1, CR3, CR4) on immune cells recognize and bind to C3b-coated targets.
|
-
C3b opsonization
-
Complement receptor-mediated phagocytosis
|
|
Inflammatory Mediator Release
|
The alternative pathway also generates several important inflammatory mediators, which play a critical role in initiating and amplifying immune responses.
|
-
C3a and C5a: These small peptides, known as anaphylatoxins, are potent inflammatory mediators that recruit and activate various immune cells
-
C5a: As a chemotactic factor
|
|
MAC Formation
|
After cleavage of C5 into C5a and C5b, the C5b fragment associates with C6, C7, C8, and multiple C9 molecules, leading to the formation of a pore in the membrane of the pathogen. This disrupts the pathogen's integrity and leads to cell lysis.
|
-
MAC formation
-
Pathogen killing
|
|
Clearance of Immune Complexes
|
The deposition of C3b on immune complexes facilitates their binding to complement receptors on phagocytic cells, promoting their clearance.
|
-
C3b-coated immune complexes are cleared
|
|
Tissue Repair and Immune Tolerance
|
By modulating the activity of various immune cells and promoting the removal of apoptotic cells and cellular debris, the alternative pathway helps maintain tissue integrity.
|
-
Tissue repair
-
Immune tolerance
|
Experimental Approaches to Study the Alternative Pathway
We explore various experimental approaches to investigate the alternative pathway of the complement system.
Complement Activation Assays
To study the activation of the alternative pathway, researchers often utilize complement activation assays that measure the formation of C3b or C3bBb complexes, as these are key intermediates in the alternative pathway.
Table 3 Techniques for complement activation assays.
|
Techniques
|
Applications
|
|
ELISA-Based Assays
|
-
Specific antibodies against C3b, C3bBb, or properdin can be used to detect their levels in plasma or serum samples. These assays provide an accurate and reproducible measure of complement activation.
|
|
Western Blotting
|
-
By using antibodies
-
against C3, C3b, C5, or other relevant proteins, researchers can determine the activation status and the relative amounts of different complement proteins in response to stimuli.
|
|
Flow Cytometry
|
-
By labeling cells with antibodies against complement components such as C3b, researchers can monitor the deposition of activated complement proteins on the cell membrane. This approach provides insight into the kinetics of complement activation and its effects on various cell types.
|
Complement-Deficient Animal Models
One of the most powerful approaches to studying the alternative pathway in vivo is the use of genetically modified animal models. Complement-deficient mice, such as those lacking C3, factor B, or factor H, can be used to assess the physiological role of the alternative pathway in health and disease.
-
C3-Deficient Mice: lack the ability to activate any of the complement pathways, allowing for a clear study of the alternative pathway in isolation.
-
Factor H-Deficient Mice: factor H-deficient mice are particularly useful for studying the regulation of the alternative pathway, as this complement regulator plays a pivotal role in controlling the amplification of the C3 convertase.
The study of the alternative pathway of the complement system is essential for understanding its role in immune defense, tissue homeostasis, and pathology. Continued innovation in experimental approaches will undoubtedly enhance our understanding of this critical immune system component and its potential as a therapeutic target in complement-related diseases.
Creative Biolabs offers a range of comprehensive complement-related products and complement testing services, including:
If you have any questions about our complement testing services and products, please feel free to contact us.
Resources
Reference
-
From Wikipedia: By Tossh_eng - Tossh_eng (Schematic illustration was made with a drawing application.), CC BY-SA 4.0 https://commons.wikimedia.org/wiki/File:Complement-pathways.png
For Research Use Only.
Related Sections: