What are Serine Proteases of Complement System?
The complement system is a proteolytic cascade, also called as complement cascade, which contains a sophisticated network of about 30 proteins circulating in serum or bound to the cell surface. There are nine serine proteases in complement system: eight of them (C1r, C1s, MASP-1, MASP-2, MASP-3, factor D, C2, factor B) are essential in both the initiation and propagation of the cascade, while factor I is a cascade-regulating protease, shown in Fig.1. Each serine protease has very restricted specificity for protein substrates and low enzymic activity. All serine proteases of complement system, except factor D, are mosaic proteins containing several noncatalytic domains preceding the catalytical serine protease domain.
The Catalytic Triad of the Serine Proteases
Proteases are enzymes that can degrade protein or peptide substrates via catalyzing the hydrolysis of the peptide bond. The important player in the catalytic mechanism of the serine proteases is the catalytic triad. The catalytic triad is located in the active site of the enzyme, where catalysis occurs and is preserved among all superfamilies of serine protease enzymes. This triad is a coordinated structure containing three amino acids: His (histidine), Ser (serine) and Asp (aspartate). These three amino acids play an essential role in the cleaving ability of the proteases.
Serine Proteases in Activation Pathways of Complement System
Complement system activation can be initiated by three pathways: classical, alternative, and lectin pathways, shown in Fig.2. Serine proteases play a crucial role in human physiology and pathology, activating each other to promote initiation and amplification of the complement cascade. The serine proteases of the complement system include C1r (85 KDa) and C1s (85 KDa) among the classical pathway (CP); MASP-1, 2, 3 (mannan-binding lectin (MBL)-associated serine proteases; 80–90 KDa) in the lectin pathway (LP); C2 (110 KDa) in the classical/lectin pathway; and Factor B (93 KDa), Factor D (25 KDa), and Factor I (88 KDa) in the alternative pathway (AP), listed in Table 1.
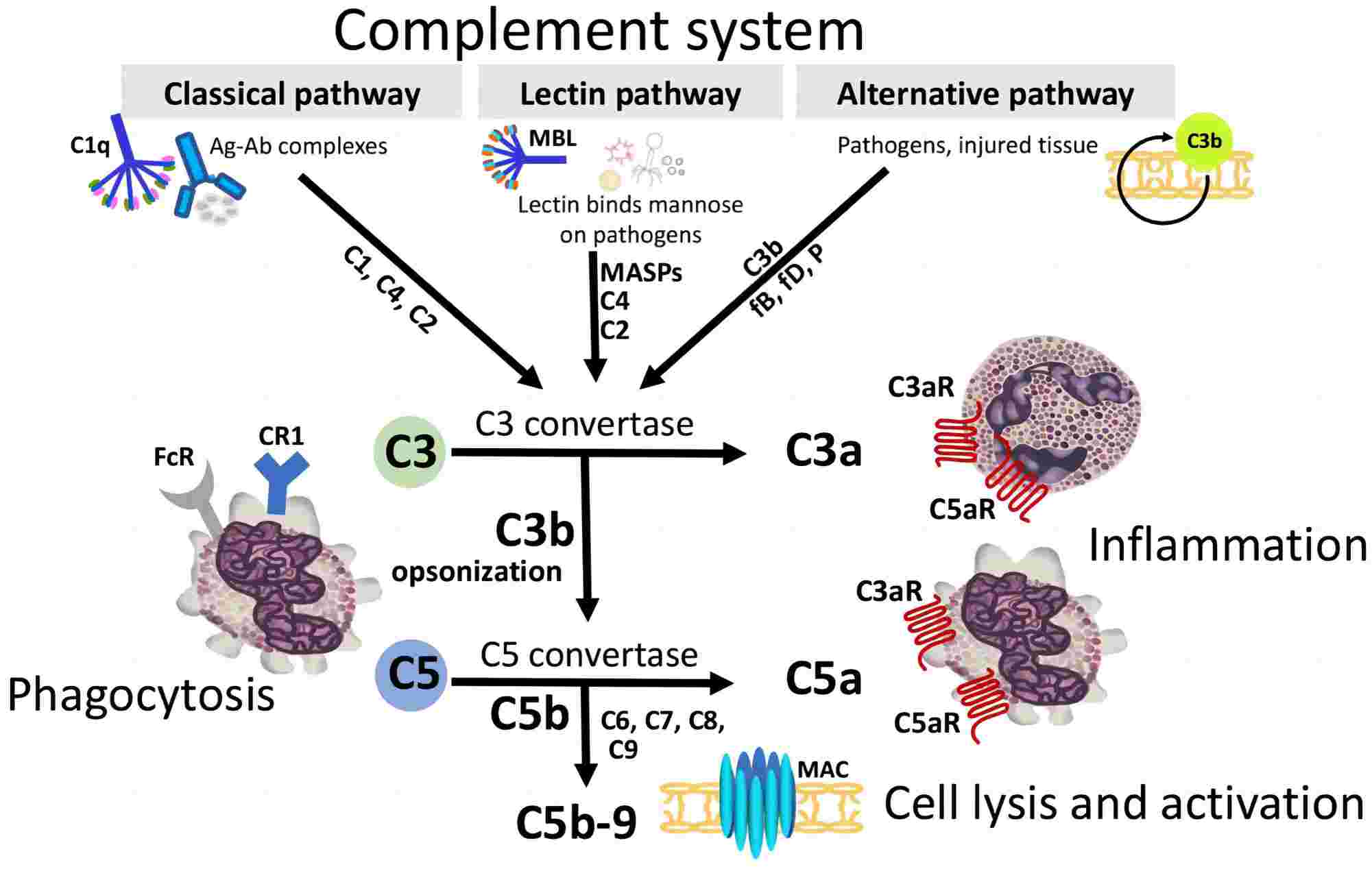
Fig. 1 Complement activation by the classical pathway (CP), lectin pathway (LP), and alternative pathway (AP).1, 2
Table.1 Serine proteases of the complement system.
|
Serine Proteases
|
Complement pathway
|
Active form
|
Function
|
|
C1r
|
Classical
|
C1 complex (C1q, C1r, C1s)
|
C1r autoactivation and C1s cleavage
|
|
C1s
|
Classical
|
C1 complex (C1q, C1r, C1s)
|
C2 and C4 cleavage
|
|
Factor I
|
Alternative
|
Factor I complex with C3b or C4b
|
C3b and C4b cleavage
|
|
Factor B
|
Alternative
|
C3bBb
|
C3 and C5 cleavage
|
|
Factor D
|
Alternative
|
C3bBD complex
|
Cleaves factor B bound to C3b
|
|
MASP-1
|
Lectin
|
MBL/MASPs complex
|
C2 (but not C4), C3 and MASP-2 cleavage and MASP-1 autoactivation
|
|
MASP-2
|
Lectin
|
MBL/MASPs complex
|
C2 and C4 cleavage
|
|
MASP-3
|
Lectin
|
MBL/MASPs complex
|
Remains unclear
|
|
C2
|
Classical/lectin
|
C4b2a
|
C3 and C5 cleavage
|
Serine Proteases Inhibitors
In terms of proteases, they play a crucial role in human physiology and pathology. Complement systems are proteolytic cascades in which serine proteases are activated from each other via limited proteolysis in the strictly ordered manner. Uncontrolled complement activation can influence and promote the development of serious disease conditions, thereby inhibition of the complement serine proteases could be a potential therapeutic method. For applying this therapeutic method, it is mainly based on serine protease inhibitors.
There are two kinds of natural serine protease inhibitors. The first one goes through major conformational change during the inhibitory process and form irreversible complexes with the target proteases. Serpins and macroglobulins are large protein inhibitors and abundant in the blood. Recently, C1-inhibitor, a natural complement protease inhibitor belonged to the serpin superfamily, which is approved for clinical use in hereditary angioedema (HAE). The second one, small protein inhibitors of serine proteases are able to form reversible complexes with the target protease via thermodynamic terms. There are two types of these tight-binding inhibitors: the canonical and noncanonical inhibitors.
References
-
Girardi, Guillermina, et al. "Essential role of complement in pregnancy: from implantation to parturition and beyond." Frontiers in immunology 11 (2020): 1681.
-
under Open Access license CC BY 4.0, without modification
Related Product
For Research Use Only.
Related Sections:

