Creative Biolabs is a leading service provider of in vitro diagnostic (IVD) antibody development. By leveraging a combination of experience and innovation in IVD antibody and assay development we can help bring your product ideas or project inception to fruition. Particularly, we offer IVD antibody development services for a potential sepsis biomarker, platelet factor 4 (PF-4).
Introduction of PF-4
PF-4 is a 70-amino acid protein and a member of the chemokine family. It is released from the alpha-granules of activated platelets and binds with high affinity to heparin. The primary physiologic role of PF-4 appears to be neutralization of heparin-like molecules on the endothelial surface of blood vessels, thereby inhibiting local antithrombin III activity and promoting coagulation. Increased levels of PF-4 are observed in a variety of clinical states that are associated with activation of platelets, including inflammatory or infectious diseases, disseminated intravascular coagulation, shock, polycythemia vera, cerebrovascular disorders, extracorporeal circulation, diabetes, cardiovascular disease, renal disease, cancer, and during the postoperative period.
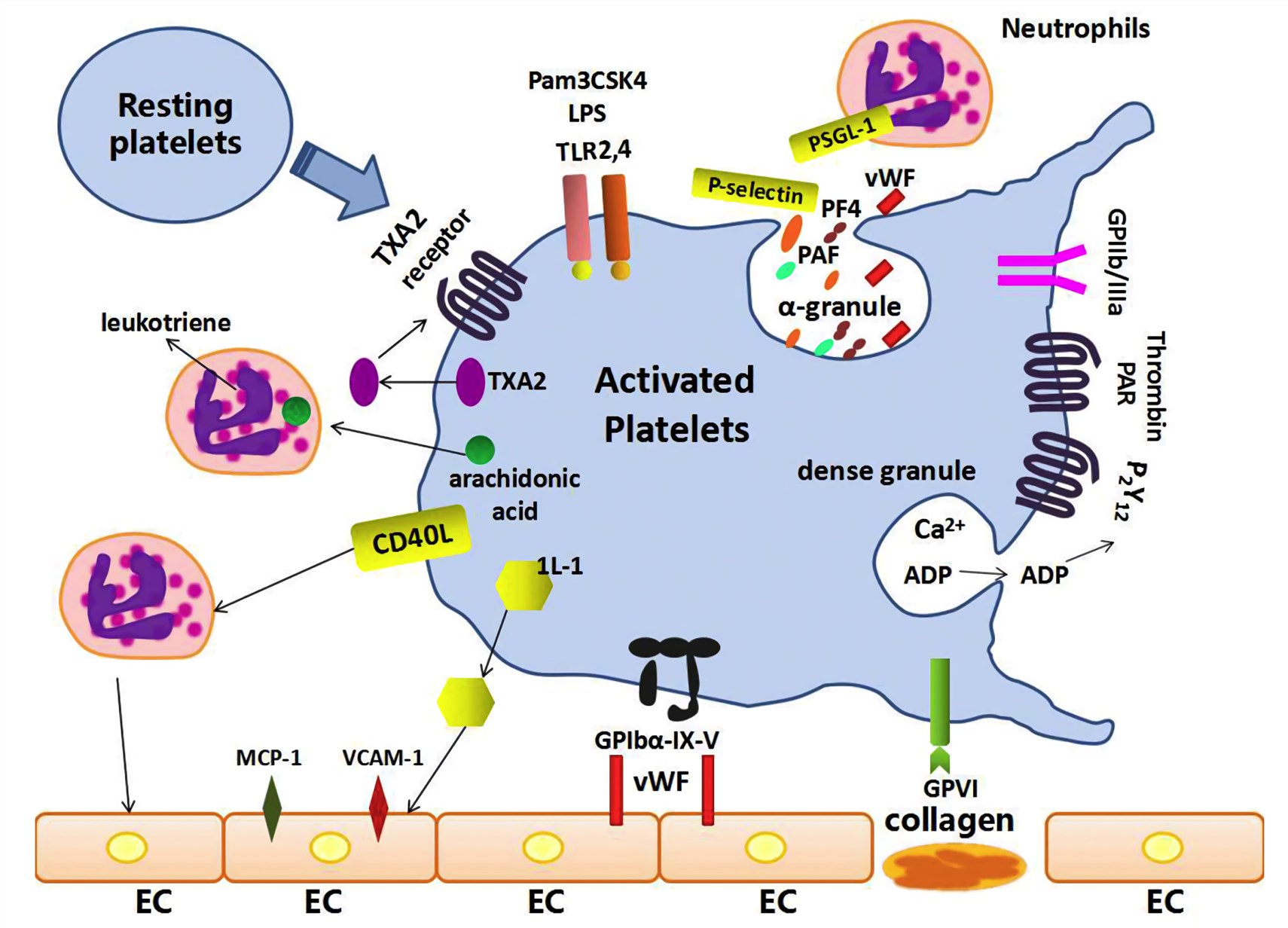 Fig.1 The mechanisms and inflammatory responses of platelet activation in sepsis. (Wang, Y., 2018)
Fig.1 The mechanisms and inflammatory responses of platelet activation in sepsis. (Wang, Y., 2018)
PF-4 for Sepsis Diagnosis
In a study on sepsis, the plasma concentrations of PF-4 were measured in ten patients with septicaemia. It is found that the PF-4 values were elevated in the acute phase and declined significantly after treatment with antibiotics and surgical therapy, suggesting the PF-4 concentration could be useful as an in vivo indicator and as a follow-up parameter of increased platelet activation. Now increasing evidence suggests that platelet activation may be an important step in the development and progression of sepsis. The fact that PF-4 is a specific marker reflecting platelet activation indicates that the measurement of this chemokine protein may be a potential clinical biomarker for aiding sepsis diagnosis. Creative Biolabs offers customized services for the development of antibodies against PF-4 biomarker to help the in vitro diagnosis of sepsis in suspected patients.
Features of IVD Antibody Development Services
- Expertise: expert in the development of antibodies for different immunoassays.
- Experience: specialized in IVD antibody and assay development with the completion of numerous IVD-related projects.
- Efficiency: short timelines to accelerate IVD product development and competitive prices with the best quality.
- Technology: an advanced technology platform to develop high-quality monoclonal, polyclonal, and recombinant antibodies; perform antigen & antibody conjugation to different markers; perform protein characterization and purification.
As an undisputed industry leader in the field of IVD antibody development, Creative Biolabs is confident in providing our clients with both high-efficient services and high-quality products. Moreover, antibodies can be developed against an extensive range of sepsis biomarkers in addition to PF-4. Flexible solutions are offered to better suit the specific needs of our clients. If you are interested in our services, contact us and we would be happy to help.
Reference
- Wang, Y., (2018). “Platelet activation and antiplatelet therapy in sepsis: A narrative review.” Thrombosis research.
For Research Use Only.