Creative Biolabs is one of the leading custom antibody generation and development providers. We specialize in the generation of antibodies against a wide range of targets for R&D, diagnostic, and therapeutic applications. Currently, we have launched a series of in vitro diagnostic (IVD) antibody development services targeting numerous diagnostic biomarkers of human diseases. Here, we focus on the F1.2 as a marker of sepsis.
Sepsis and Disseminated Intravascular Coagulation
Disseminated intravascular coagulation (DIC) is a complication of many diseases such as sepsis, cancer, and trauma. Several factors contribute to the development of DIC, including aberrations in the endothelium and altered levels of various endogenous procoagulant, anticoagulant, and fibrinolytic factors. Studies have reported that the frequency of DIC in sepsis patients is 25%-50%. To some extent, the identification of DIC might be a reliable method for the early diagnosis of sepsis. The identification of DIC is mainly based on the results of several blood tests, including prothrombin time, platelet count, fibrinogen levels, and various other tests that measure the activation of coagulation and the fibrinolytic system. Newer methods detect the D-dimer, thrombin-antithrombin complexes (TAT), fibrinopeptides, and F1.2 in blood samples from patients.
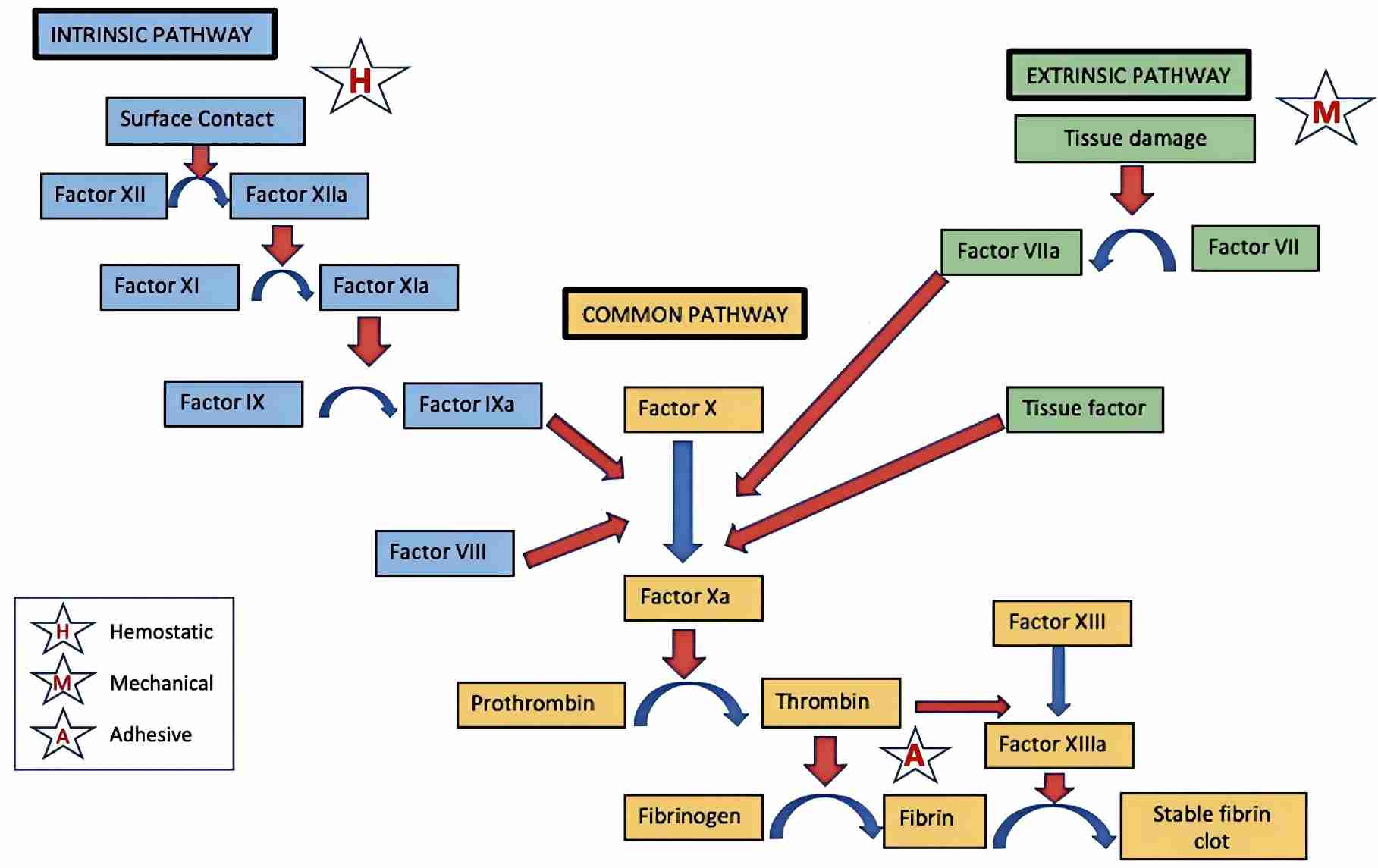 Fig. 1 Coagulation pathway.1
Fig. 1 Coagulation pathway.1
F1.2 for Sepsis Prognosis & Diagnosis
The conversion of prothrombin to thrombin is a key event in the coagulation of blood. When prothrombin is converted to thrombin, F1.2 is released from prothrombin. In a study on sepsis, plasma markers of protein C activation (e.g., F1.2, activated protein C levels) were measured in both septic patients and in healthy volunteers. The findings showed that all patients had elevated F1.2 levels. Moreover, the baseline F1.2-to-APC rations may have prognostic value in predicting clinical outcome. As a result, F1.2 might be one of the markers that can be used to identify patients with sepsis.
Features of IVD Antibody Development Services
- Expertise: expert in the development of antibodies for different immunoassays (e.g., ELISAs, immunohistochemistry, Western blot, immunochromatography assay).
- Experience: years of experience in IVD antibody and assay development with the completion of numerous IVD-related projects.
- Efficiency: short timelines to accelerate IVD product development.
- Facility: fully equipped facility to perform antigen & antibody conjugation to different markers; different methods for protein characterization and purification.
Creative Biolabs provides IVD antibody development services against different sepsis biomarkers, in addition to F1.2, to help researchers in the development of different immunoassays and IVD kits. Supported by our highly specialized scientists and advanced platform, we are confident in providing the best services and high-quality products at the most competitive prices. If you are interested in our services, contact us for more information.
Reference
- Dammann, Kyle, et al. "Operative Hemostasis in Trauma and Acute Care Surgery: The Role of Biosurgical Agents." (2020).
For Research Use Only.