Antisense Oligonucleotides (ASOs) Products for Cancer
As a recognized world leader biotechnology company, Creative Biolabs offers high-quality ASO products for cancer therapy. A variety of genes have been validated as molecular targets for antisense therapy, such as the key regulators of cell growth, apoptosis, angiogenesis, and metastasis that are associated with the malignant phenotype of cancer cells rather than with normal cell physiology.
Antisense Oligonucleotides (ASOs) Overview
Antisense oligonucleotides (ASOs) have emerged as an exciting and promising strategy for cancer therapy. The antisense technology is based on the sequence-specific binding of an antisense oligonucleotide to target mRNA, resulting in the prevention of gene translation. Antisense therapeutics offers one approach to target genes that are associated with the pathogenesis of human cancers, especially those that are not amenable to small-molecule or antibody inhibition. Clinical studies have shown that the treatment with ASOs is safe and leads to target downregulation in tumor tissues.
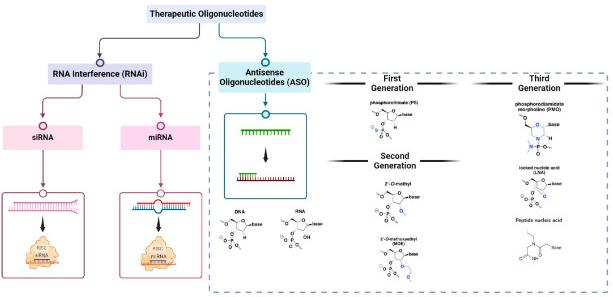 Figure 1 Therapeutic oligonucleotides modalities: RNA interference (RNAi) and antisense oligonucleotides (ASOs).1
Figure 1 Therapeutic oligonucleotides modalities: RNA interference (RNAi) and antisense oligonucleotides (ASOs).1
Antisense Oligonucleotide for Cancer
Cancer is a complex disease characterized by uncontrolled cell growth, evasion of apoptosis, enhanced angiogenesis, and metastasis. Many genes play crucial roles in these processes, and antisense oligonucleotides (ASOs) provide a targeted approach to regulate the expression of these genes.
Key Molecular Targets in Cancer
| Target Category | Specific Targets | Cancer Types | Mechanism of Action |
|---|---|---|---|
| Anti-apoptotic proteins | Bcl-2, Bcl-xL, Mcl-1 | Lymphoma, leukemia, solid tumors | Restore apoptosis |
| Oncogenic transcription factors | c-Myc, STAT3, HIF-1α | Various carcinomas | Inhibit proliferation and metabolism |
| Growth factor receptors | EGFR, HER2, IGFR | Breast, lung, colorectal cancers | Downregulate receptor expression |
| Cell cycle regulators | Cyclins, CDKs, Aurora kinases | Various malignancies | Cell cycle arrest |
| Non-coding RNAs | miRNAs, lncRNAs | Various cancers |
Antisense Oligonucleotide Therapy
Antisense oligonucleotide therapy seeks to restore or alter the expression of specific genes that have gone awry in diseases such as cancer. Anti sense oligonucleotides can work via multiple different pathways.
-
Ribonuclease H-mediated Decay
Binding of an antisense oligonucleotide to its target mRNA can direct ribonuclease H (RNase H), an endonuclease that cleaves RNA strands of RNA-DNA hybrids, to the target mRNA leading to its degradation and inhibiting translation. -
Direct Steric Blockage
Anti sense oligonucleotides can also work by directly blocking ribosome binding sites or other elements on the mRNA, or by interfering with other steps in translation. The production of target proteins can be inhibited by directly blocking the translation machinery. -
Exon Content Modulation
For pre mRNA, antisense oligonucleotides can bind to splicing sites and alter the splicing process and the exon content of the mature mRNA, thereby allowing for the correction of aberrant splicing events that are disease and cancer specific.
Antisense Oligonucleotide Remains in Plasma
The plasma stability of ASO is a key determinant of its efficacy, as unmodified oligonucleotides are rapidly degraded by nucleases in blood and tissues. ASO achieved further stability through 2 '- glycosylation modifications, including 2'-oxymethyl (2'-OMe), 2'- oxymethoxyethyl (2'-MOE), and 2'-fluoro (2'-F) substitutions. These modifications not only enhance nuclease resistance, but also improve the binding affinity with complementary RNA sequences by stabilizing the glycosylation folding in the C3 '- internal conformation, which is the preferred conformation for RNA-RNA and RNA-DNA hybridization.
Pharmacokinetic Properties of Chemically Modified ASOs
| Parameter | Unmodified ASO | PS-Modified ASO | 2'MOE-Modified ASO | LNA-Modified ASO |
|---|---|---|---|---|
| Plasma half-life | <10 minutes | 24-48 hours | 1-4 weeks | 2-5 weeks |
| Protein binding | Low | High (>90%) | High (>90%) | High (>90%) |
| Renal clearance | Rapid | Reduced | Significantly reduced | Significantly reduced |
| Tissue distribution | Limited | Widespread | Widespread | Widespread |
| Metabolic pathway | 3'-exonuclease | 3'-exonuclease | Endonuclease | Endonuclease |
Antisense Oligonucleotide Toxicity
ASO toxicity is a key factor in its clinical development. Off target effects, where ASO binds to unexpected mRNA, may lead to unnecessary gene silencing. This can be alleviated through careful design and screening of AMPLATZER septal occluders. More severe toxicity usually comes from chemical modifications themselves. Thiophosphate modified ASOs can interact with plasma proteins, leading to complement activation, and are associated with dose limiting toxicity such as thrombocytopenia and nephrotoxicity.
Strategies to reduce the toxicity of antisense oligonucleotides (ASOs) include:
- Chemical optimization: Introducing 2 '- O-methyl and 2' - MOE modifications can reduce immune activation and enhance specificity while maintaining nuclease resistance.
- Tissue-specific delivery: Binding with GalNAc can promote liver cell specific uptake, reduce kidney exposure, and lower systemic dose of liver targets.
- Sequence optimization: Advanced algorithms can evaluate potential off target interactions and secondary structures that may increase toxicity.
- Medication regimen adjustment: Extending the dosing interval helps to recover from sub toxic effects, while a lower initial dose and gradually increasing the dose helps to determine individual tolerance.
Frequently Asked Questions
Q: What are the differences between the first, second, and third generation ASOs?
A: The first generation ASO mainly involves skeleton modification, such as thiophosphate modification, which can improve distribution and reduce urine excretion. The second generation ASO focuses on glycosylation modification, enhancing binding affinity and reducing degradation. The third-generation ASO has been modified with phosphate groups, sugar moieties, and nucleobases to enhance binding affinity, cell penetration, and reduce off target effects.
Q: Can ASO be used to treat all types of cancer?
A: Although ASO has the potential to treat various types of cancer, not all cancers are currently suitable for ASO based therapies.
Q: How is ASO delivered to cancer cells in the body?
A: ASO can be delivered through various methods. Thiophosphate ASO can sometimes be delivered to cells without the need for a delivery carrier. In some cases, ASO can be injected into the cerebrospinal fluid to distribute it along the neural axis. Systems based on nanocarriers are also being explored to improve the delivery efficiency of ASO to cancer cells.
Q: What are the main limitations of current ASO delivery methods in solid tumors?
A: The main limitations include uneven vascular perfusion, increased interstitial pressure, dense extracellular matrix, and cellular heterogeneity within the tumor. These factors constitute physical and biological barriers, limiting the uniform distribution of ASO throughout the entire tumor mass.
Q: Can anti apoptotic inhibitors (ASOs) be used in combination with conventional chemotherapy or targeted therapy?
A: Yes, ASO can be used in combination with other cancer therapies and has significant effects. For example, ASOs targeting anti apoptotic proteins can enhance the sensitivity of tumor cells to chemotherapy, while ASOs targeting resistance mechanisms can restore the sensitivity of tumor cells to targeted drugs.
Connect with Us Anytime!
As a recognized world leader biotechnology company, Creative Biolabs offers high-quality ASO products and synthesis services for cancer therapy. A variety of genes have been validated as molecular targets for antisense therapy, such as the key regulators of cell growth, apoptosis, angiogenesis, and metastasis that are associated with the malignant phenotype of cancer cells rather than with normal cell physiology. Our platform has developed abundant antisense products targeting the mRNA of Bcl-2, Protein kinase C (PKC), c-raf or Ha-ras, etc. In addition, we are also committed to developing other new potential targets for specific antisense treatment of cancer. Please feel free to contact us by e-mail for a quote and further discussion with our scientists.
Reference
- Khuu A, Verreault M, Colin P, et al. Clinical applications of antisense oligonucleotides in Cancer: a focus on Glioblastoma. Cells, 2024, 13(22): 1869. https://doi.org/10.3390/cells13221869 (Distributed under Open Access license CC BY 4.0, without modification.)
