A Novel TfR-Targeting Conjugate for Brain Drug Delivery
Introduction
The blood-brain barrier (BBB), a specialized neurovascular unit that stringently regulates molecular traffic into the brain, fundamentally constrains the development of effective pharmacotherapies for central nervous system (CNS) disorders. Consequently, systemic administration of macromolecules like therapeutic antibodies and nucleic acids results in minimal CNS penetration, with brain-to-blood exposure ratios often below the sub-therapeutic level of 0.1. To circumvent this delivery challenge, one prominent strategy involves co-opting endogenous transport systems, particularly receptor-mediated transcytosis (RMT), to shuttle therapeutic agents across the BBB. In this context, aptamers are being investigated as novel targeting ligands. These synthetic oligonucleotides fold into unique structures, allowing them to bind targets with an affinity rivaling monoclonal antibodies but with distinct advantages, including a smaller size and simpler chemical synthesis. It is speculated, therefore, that these characteristics make aptamers well-suited for engaging RMT pathways, offering a promising platform to enhance CNS drug penetration.
In this study, a team of scientists developed and validated a novel conjugate designed for superior brain delivery. Their work involved the discovery of a new RNA aptamer that specifically targets the human transferrin receptor (TfR), a key transporter at the BBB. This aptamer was chemically conjugated to a DSPE-PEG lipid to enhance its lipophilicity and stability, and then linked to either a fluorescent dye for imaging or a therapeutic DNA oligonucleotide (an anti-miR). Through a series of in vitro and in vivo experiments, the scientists demonstrated that this tripartite conjugate effectively crossed the BBB in mice, delivering its payload directly to neurons.
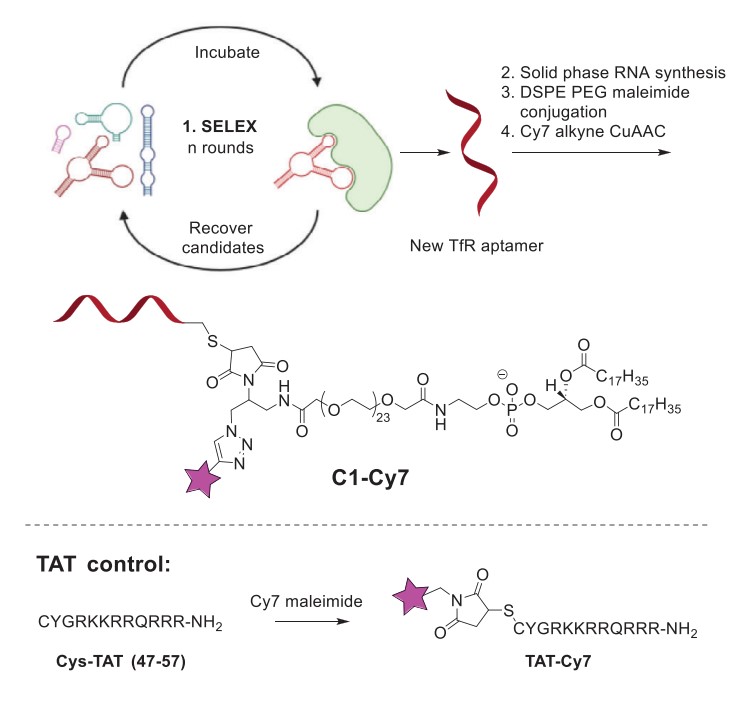 Fig.1 Design and synthesis of C1-Cy7 and TAT-Cy7.1
Fig.1 Design and synthesis of C1-Cy7 and TAT-Cy7.1
Design, Synthesis, and Biochemical Characterization of the Conjugate
The conjugate's design began with the discovery of a novel TfR-targeting RNA aptamer using the Systematic Evolution of Ligands by Exponential Enrichment (SELEX). This process yielded an 87-nucleotide aptamer which was subsequently truncated to a more efficient 22-nucleotide functional unit. The truncated aptamer demonstrated exceptionally high binding affinity to the human TfR protein, with an equilibrium dissociation constant (KD) of 2.20 x 10-11 M, confirming its high specificity. For synthesis, the aptamer was produced with a 5'-thiol modification. This allowed for a precise bioconjugation reaction with a custom DSPE-PEG-maleimide reagent, forming an aptamer-lipid intermediate (C1). This intermediate was then "clicked" to either a Cy7-alkyne for imaging studies or an anti-miR-125b1-alkyne for therapeutic efficacy studies using copper-catalyzed azide-alkyne cycloaddition (CuAAC). Biochemical analysis confirmed the success of the conjugation and revealed that the final conjugate (C1) had a serum half-life of 3.9 hours, a nearly four-fold improvement over the 1.0-hour half-life of the unconjugated aptamer, highlighting the stabilizing effect of the lipid component.
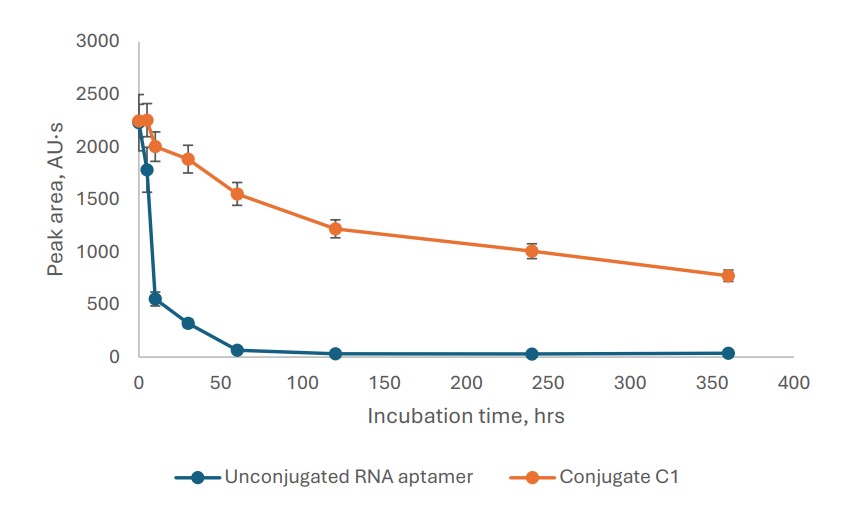 Fig.2 Half-lifetime study of RNA aptamer and C1 conjugate in mouse serum.1
Fig.2 Half-lifetime study of RNA aptamer and C1 conjugate in mouse serum.1
In Vitro Efficacy and Barrier Penetration Studies
To assess the conjugate's ability to cross the BBB and exert a biological effect, the researchers performed two key in vitro assays:
- Cellular Penetration Assay: Using a co-culture model of the human BBB (hCMEC/D3 cells grown over neuroblastoma cells), the fluorescently labeled conjugate (C1-Cy7) demonstrated high passage efficacy through the cellular barrier, outperforming a free Cy7 dye and showing superior passage compared to the well-known cell-penetrating peptide TAT.
- Therapeutic Efficacy Assay: This assay utilized the conjugate armed with a therapeutic anti-miR-125b1 payload (C2). When applied to human neuroblastoma cells, which express high levels of the target miR-125b1, the C2 conjugate induced a dose-dependent reduction in cell viability (34% reduction at 450 nM). Conversely, in control cells (BEAS-2B) with low miR-125b1 expression, viability was only minimally affected (13% reduction), confirming that the conjugate's cytotoxic effect was target-specific.
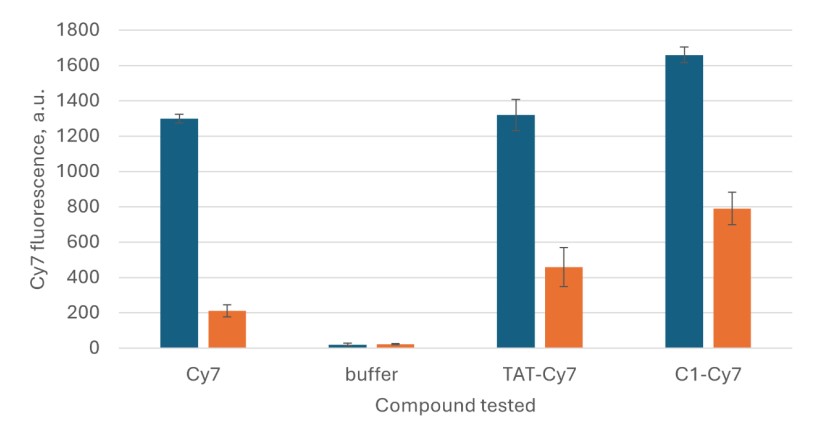 Fig.3 Results of hCMEC/D3 passage tests for TAT-Cy7 and C1-Cy7.1
Fig.3 Results of hCMEC/D3 passage tests for TAT-Cy7 and C1-Cy7.1
In Vivo Biodistribution, Efficacy, and Pharmacokinetic Studies
The performance of the conjugate was further evaluated in Balb/cJ mice. Longitudinal whole-body fluorescence imaging revealed that systemically administered C1-Cy7 effectively accumulated in the brain, whereas a TAT-Cy7 control primarily localized to the liver. This brain-targeting capability was confirmed with the therapeutic C2 conjugate; qPCR analysis of harvested organs showed that the concentration of the anti-miR DNA payload was significantly higher in the brain than in the liver or kidney. Pharmacokinetic analysis revealed a remarkably high brain-to-serum ratio for the delivered oligonucleotide, peaking at 4.6-6.5 within 48 hours post-injection, a result without precedent for similar delivery systems. Furthermore, fluorescence in situ hybridization (FISH) imaging of brain tissue confirmed that the conjugate's signal was located within neurons and not merely retained within the blood vessels, proving it had successfully crossed the BBB and engaged its target cells.
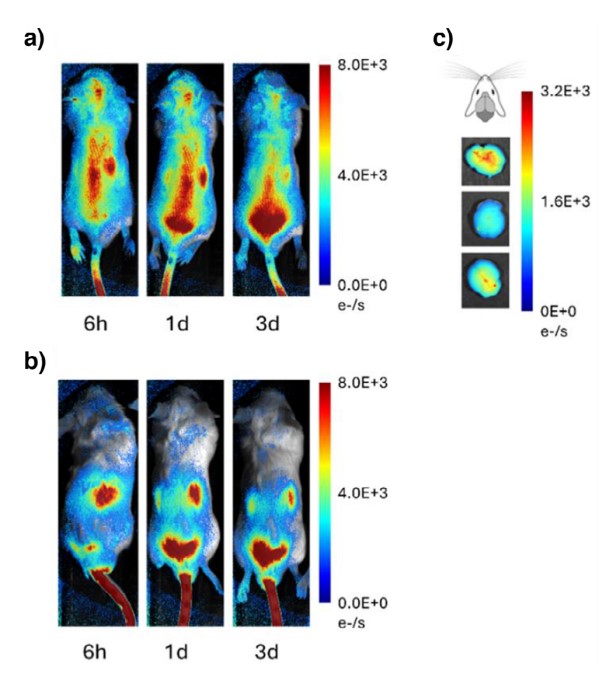 Fig.4 Whole-body fluorescence images of C1-Cy7 (a) and TAT-Cy7 (b), and a brain image 1 day after C1-Cy7 injection (c).1
Fig.4 Whole-body fluorescence images of C1-Cy7 (a) and TAT-Cy7 (b), and a brain image 1 day after C1-Cy7 injection (c).1
Toxicology Assessment
A critical component of the study was the safety assessment of the conjugate. One week after a single intravenous dose, a comprehensive histopathological analysis was performed on H&E-stained sections of the brain, kidney, liver, spleen, and lungs. The results showed no signs of acute toxicity for the C1-Cy7 conjugate. Specifically, the brain tissue of treated mice showed no evidence of inflammation, gliosis, or neuronal loss. This favorable safety profile stood in sharp contrast to the TAT-Cy7 control group, which exhibited significant pathological changes, particularly inflammation and fibrosis in the kidneys and lungs. These findings underscore the superior safety and tolerability of the novel TfR aptamer-lipid conjugate design.
 Fig.5 Histopathologic analysis of brain tissue in H&E-stained mouse.1
Fig.5 Histopathologic analysis of brain tissue in H&E-stained mouse.1
This study demonstrates a powerful new platform for brain drug delivery. By combining a specific RNA aptamer targeting the transferrin receptor with a stabilizing lipid, the researchers created a conjugate that transports an oligonucleotide payload across the blood-brain barrier with unparalleled efficiency and safety. The high brain-to-serum ratio and confirmed neuronal uptake mark a significant advance in overcoming the critical delivery challenges that have hindered therapies for neurological diseases.
At Creative Biolabs, we specialize in the complex chemistry that makes such breakthroughs possible. Our team provides expert oligonucleotide-related conjugation services, helping our clients design and synthesize the next generation of targeted therapeutics. If you are working to deliver a novel payload to the brain or another challenging tissue, contact us to explore how our expertise can advance your program.
Reference
- Song, Min‐sun, et al. "New Transferrin Receptor‐Targeting Conjugate Effectively Delivers DNA to Mouse Brain." Angewandte Chemie International Edition (2025): e202500247. Under open access license CC BY 4.0, without modification.
Related Services
- Custom Protein-Oligonucleotide Conjugation Service
- Custom Oligonucleotides-Peptide Conjugation Service
- Custom Oligonucleotides-Bead & Particle Conjugation Service
- Custom Oligonucleotides-Small Molecule Conjugation Service
