Advancing Cancer Immunotherapy Through Targeted Treg Modulation
The Challenge of the Immunosuppressive Tumor Microenvironment
The tumor microenvironment (TME) presents a formidable obstacle in cancer treatment, largely by fostering an immunosuppressive landscape that allows malignant cells to evade immune destruction. Central to this immune evasion are regulatory T cells (Tregs), a specialized T cell subset that actively dampens anti-cancer immune responses, thereby diminishing the effectiveness of various immunotherapies. While broadly depleting Tregs might appear beneficial, such systemic approaches often trigger undesirable immunotherapy-related adverse events (irAEs), highlighting the necessity for strategies that can precisely target and modulate Tregs within the confines of the TME. Drug conjugates, meticulously engineered as "guided missiles," offer such a sophisticated solution. These constructs typically integrate a cytotoxic or immunomodulatory payload with a carrier molecule designed to recognize specific markers on Tregs, all connected via a specialized linker system. This architecture enables the selective delivery of the therapeutic agent directly to the target Tregs, aiming to maximize therapeutic efficacy while substantially reducing systemic toxicity and off-target effects, thus heralding a new era of more effective and safer cancer treatments.
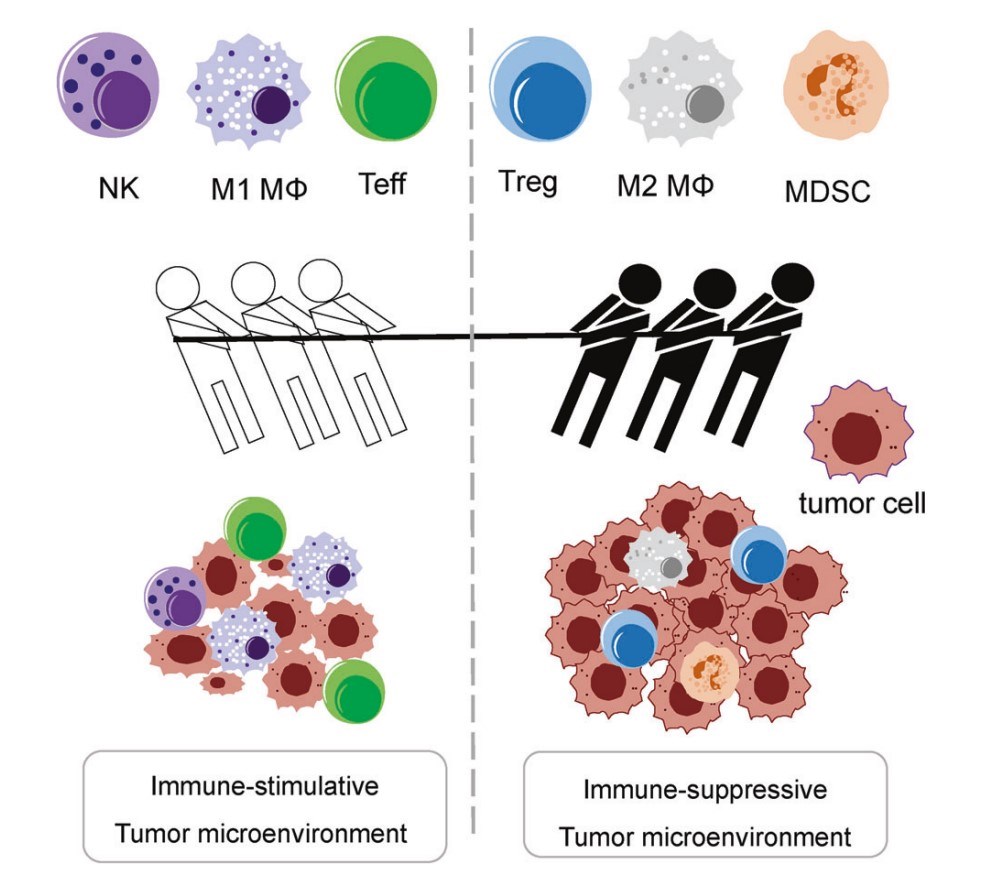 Fig.1 The mutual dynamics between the immunostimulatory and immunosuppressive TME.1
Fig.1 The mutual dynamics between the immunostimulatory and immunosuppressive TME.1
Regulatory T Cell Mechanisms and Key Molecular Targets in the TME
Tregs accumulate significantly within the TME, drawn by chemokine gradients secreted by tumor cells and other stromal components. Once recruited, they deploy a multifaceted arsenal of immunosuppressive mechanisms. These include the competitive blockade of costimulatory signals on antigen-presenting cells (APCs) via CTLA-4 (Cytotoxic T-Lymphocyte-Associated protein 4), the secretion of immunosuppressive cytokines like IL-10 and TGF-β, the depletion of local IL-2 pools crucial for effector T cell proliferation due to their high expression of CD25 (the alpha chain of the IL-2 receptor), and even the direct killing of effector T cells and APCs through perforins and granzymes. The strategic targeting of these tumor-infiltrating Tregs relies on identifying and exploiting specific cell surface and intracellular markers. Key surface markers include the aforementioned CD25 (a hallmark of activated effector Tregs), CTLA-4, and other immune checkpoints like PD-1. Additionally, costimulatory receptors such as GITR (Glucocorticoid-Induced TNFR-related protein), OX40, and ICOS, which are often upregulated on Tregs, serve as potential targets. Chemokine receptors like CCR4 and CCR8, which mediate Treg trafficking to the tumor site, are also attractive for selective targeting. Intracellularly, the transcription factor FOXP3 is considered the master regulator defining Treg lineage and function, making it a critical, albeit more challenging, target.
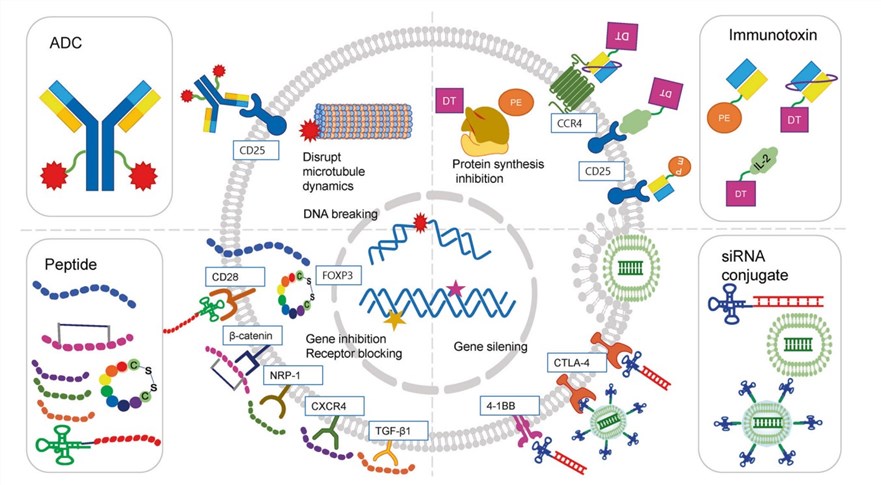 Fig.2 Illustration of the action mechanism of drug conjugates targeting Tregs.1
Fig.2 Illustration of the action mechanism of drug conjugates targeting Tregs.1
Antibody-Drug Conjugates (ADCs)
ADCs are a clinically advanced class of targeted therapeutics. They consist of three main components: a monoclonal antibody (mAb) engineered for highly specific binding to a target cell's surface antigen, a potent cytotoxic payload, and a chemical linker connecting the antibody to the payload. Upon binding to the target cell, the ADC is typically internalized, and the payload is released intracellularly through linker cleavage or degradation of the ADC, leading to cell death. For targeting Tregs in the TME, ADCs are designed against specific Treg surface markers. Key examples include:
- CD25: A prominent target due to its high expression on activated effector Tregs in the TME, which are primary mediators of immunosuppression. ADCs targeting CD25 can selectively deplete these Treg populations to restore anti-tumor immunity.
- CTLA-4: This highly expressed immune checkpoint receptor on Tregs is crucial for their suppressive function, primarily by inhibiting T cell activation through competition with CD28 for CD80/CD86 binding. ADCs targeting CTLA-4 can deliver a cytotoxic payload to Tregs and potentially interfere with this suppressive pathway, aiming to eliminate or impair these cells.
- CCR4 (C-C Chemokine Receptor Type 4): Preferentially expressed on effector Tregs, this receptor is key for their migration to and accumulation within the TME via CCL17 and CCL22 ligands. ADCs targeting CCR4 aim to eliminate Tregs as they traffic to or reside in the tumor, reducing local immunosuppression. Its selective expression on tumor-infiltrating Tregs over peripheral or effector T cells offers a therapeutic window.
Peptide-Drug Conjugates (PDCs)
PDCs are an evolving therapeutic modality. They primarily consist of a homing peptide for specific cell recognition, a therapeutic payload (which can be cytotoxic or modulatory), and a linker connecting these two components. PDCs offer potential advantages over larger antibody-based conjugates, such as improved tumor penetration due to their smaller size and potentially lower immunogenicity. The homing peptide can be a cell-targeting peptide (CTP) designed to bind with high affinity to receptors overexpressed on target cells, or a cell-penetrating peptide (CPP) that facilitates the translocation of the conjugate across cell membranes to reach intracellular targets. While the development of PDCs with directly cytotoxic payloads for Treg depletion is an active area of research, many current peptide-based strategies targeting Tregs focus on modulating their function rather than causing immediate cell death. Key approaches include:
- Targeting Intracellular Master Regulators (e.g., FOXP3): Peptides such as P60 (and its optimized cyclic version CM-1315) are designed to enter Tregs and interfere with the function of FOXP3, the master transcription factor for Treg development and suppressive activity. This interference can occur by preventing the nuclear translocation of FOXP3 or its DNA-binding activity. These peptides can sometimes be conjugated to T-cell targeting moieties, like CD28 aptamers, to enhance specificity.
- Disrupting Key Signaling Pathways: Other peptides aim to interrupt critical signaling pathways essential for Treg function or maintenance. This includes peptides that target the β-catenin/BCL9 interaction, which is involved in Treg generation, or peptides that inhibit TGF-β signaling, a major immunosuppressive cytokine produced by and acting on Tregs.
- Antagonizing Treg Surface Receptors: Peptides are also being developed to antagonize specific receptors on Tregs that contribute to their suppressive phenotype or survival, such as NRP-1 (Neuropilin-1) or the chemokine receptor CXCR4. By blocking these receptors, peptide antagonists can inhibit Treg proliferation or function.
Small Interfering RNA (siRNA) Conjugates
siRNA conjugates are an innovative therapeutic class employing RNA interference (RNAi) for targeted gene silencing. These conjugates consist of a siRNA molecule (a short, double-stranded RNA complementary to a target mRNA) and a delivery system or targeting ligand (e.g., aptamers, nanoparticles) crucial for in vivo protection and specific cell uptake. Upon internalization, the siRNA duplex unwinds, and its guide strand integrates into the RNA-induced silencing complex (RISC). This complex then uses the guide strand to bind and cleave the complementary mRNA, preventing protein translation and effectively "silencing" the target gene. To target Tregs in the TME, siRNA conjugates aim to silence genes essential for Treg development, survival, or immunosuppressive functions, thereby diminishing their population or activity. Key genetic targets within Tregs include:
- STAT3 (Signal Transducer and Activator of Transcription 3): A critical transcription factor involved in the differentiation and function of Tregs. Silencing STAT3 can inhibit Treg development and reduce their immunosuppressive capacity.
- CTLA-4: As a key checkpoint molecule mediating Treg suppressive function, silencing the CTLA4 gene aims to directly reduce one of the primary mechanisms by which Tregs inhibit effector T cell responses.
- FOXP3: The master transcriptional regulator defining Treg lineage and function. Downregulating FOXP3 expression is a strategy to destabilize Treg identity and suppressive capabilities.
- PD-1: While also known as a target on effector T cells, PD-1 is expressed on Tregs and can contribute to their function. Silencing PDCD1 (the gene encoding PD-1) in Tregs is another potential avenue to modulate their activity within the TME.
Tregs in the TME is a pivotal advancement in cancer immunotherapy, addressing the significant barrier of Treg-orchestrated immunosuppression. Drug conjugate technologies described above offer diverse and precise mechanisms to selectively deplete or incapacitate these cells. Neutralizing these key architects of immune suppression at the tumor site aims to reprogram the TME, unleash potent anti-tumor immune responses, and improve patient outcomes. Ongoing refinement of these platforms, driven by deeper Treg biology insights and molecular engineering advancements, holds immense promise for developing more potent and safer immunotherapeutic strategies.
Our Services
To support the pioneering research and development efforts in this exciting field, Creative Biolabs offers a suite of specialized services. Our expertise includes advanced ADC development, bespoke PDC conjugation, and sophisticated oligonucleotide conjugation services. We are committed to empowering the scientific community in creating the next generation of targeted cancer immunotherapies that can effectively overcome immune suppression and transform patient care. Please contact us to discuss your project requirements and explore potential collaborations.
Reference
- Yang, Juwon, and Hyunsu Bae. "Drug conjugates for targeting regulatory T cells in the tumor microenvironment: guided missiles for cancer treatment." Experimental & Molecular Medicine 55.9 (2023): 1996-2004. Under open access license CC BY 4.0, without modification.
Related Services
- Custom Protein-Small Molecule Conjugation Service
- Custom Oligonucleotides-Small Molecule Conjugation Service
- Custom Small Molecule-Particle Conjugation Service
- Custom Small Molecule-Liposome Conjugation Service
