Specific Bioconjugation of N- & C-terminal Cysteines
In protein bioconjugation, site-specific modifications play a crucial role in generating homogeneous protein bioconjugates with superior properties compared to heterogeneous forms. The limited abundance of cysteine (Cys) and the reactivity of their thiol side chains make them particularly valuable for these targeted conjugation strategies. Additionally, the N- and C-termini of proteins are prime candidates for site-specific modifications due to their solvent accessibility and the unique single occurrence in single-chain proteins. Protein-terminal Cys can provide additional specificity, which allows for the generation of stable conjugates at the ends of various proteins via terminal amine groups as well as side-chain thiol groups.
Incorporation of Cys at the Protein Terminus
Before conjugation, it is essential to introduce Cys at the terminus of the target protein. For N-terminal cysteine (NCys), this is typically achieved by adding peptide tags to the N-terminus of the protein, which can then be cleaved to yield the desired NCys. Enzymes such as methionine aminopeptidases (MetAPs), tobacco etch virus (TEV) proteases, and prolyl aminopeptidases (ProAPs) effectively cleave specific peptide sequences to generate NCys. In contrast, C-terminal Cys (CCys) is introduced via site-directed mutagenesis.
 Fig.1 Examples of NCys installation.1, 2
Fig.1 Examples of NCys installation.1, 2
NCys Conjugations
The conjugation methods targeting NCys primarily include several approaches, as outlined in Table 1.
Table 1 Methods for NCys bioconjugation.1, 2
| Strategies | Reactions and Conditions | Mechanisms |
| Native chemical ligation (thioester reagents) |
|
The amine group of NCys can react with the thioester to form an amide bond. |
| 2-Cyanobenzothiazole (CBT) |
|
The cyano group of CBT reacts with the 1,2-aminothiol of NCys (click condensation reaction) to form a luciferin-type product. |
| N,S-Dual labeling (CBT and maleimide) |
|
The opening of the 2-aminothiazolidine ring during the addition of CBT leads to the formation of amidine. Subsequent modification of the liberated thiol can be carried out by reaction with maleimide. The Cys-Ile-Ser tripeptide motif facilitates amidine formation. |
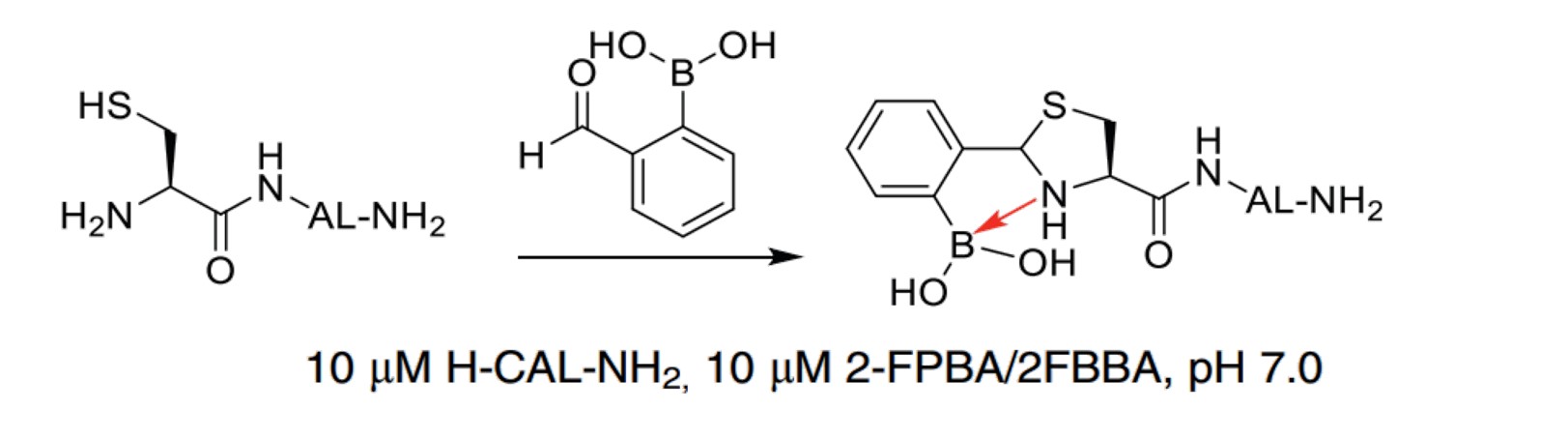
| Aromatic aldehydes with an o-boronic acid (e.g., 2-formylphenylboronic acid (2-FPBA)) |
|
2-FPBA reacts with NCys to form a thiazolidino boronate (TzB) complex. The boronic acid portion of 2-FPBA accelerates thiazolidine formation. |
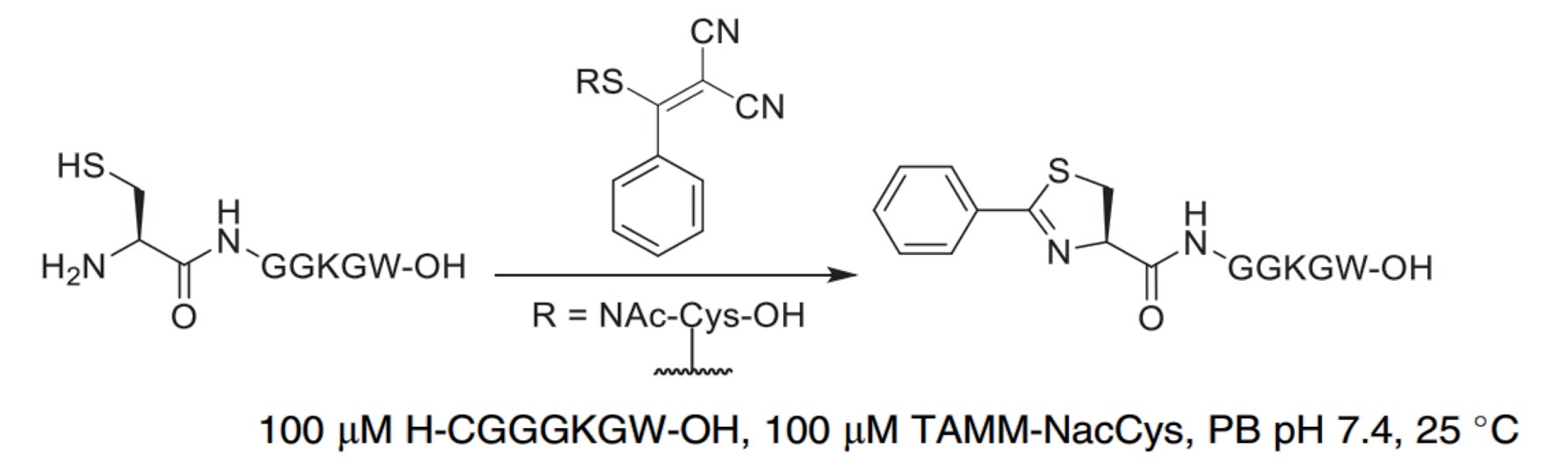
| 2- ((alkylthio) (aryl)methylene)malononitrile (TAMM) |
|
NCys undergoes a condensation reaction with TAMM to form a 2-aryl-4,5-dihydrothiazole. |
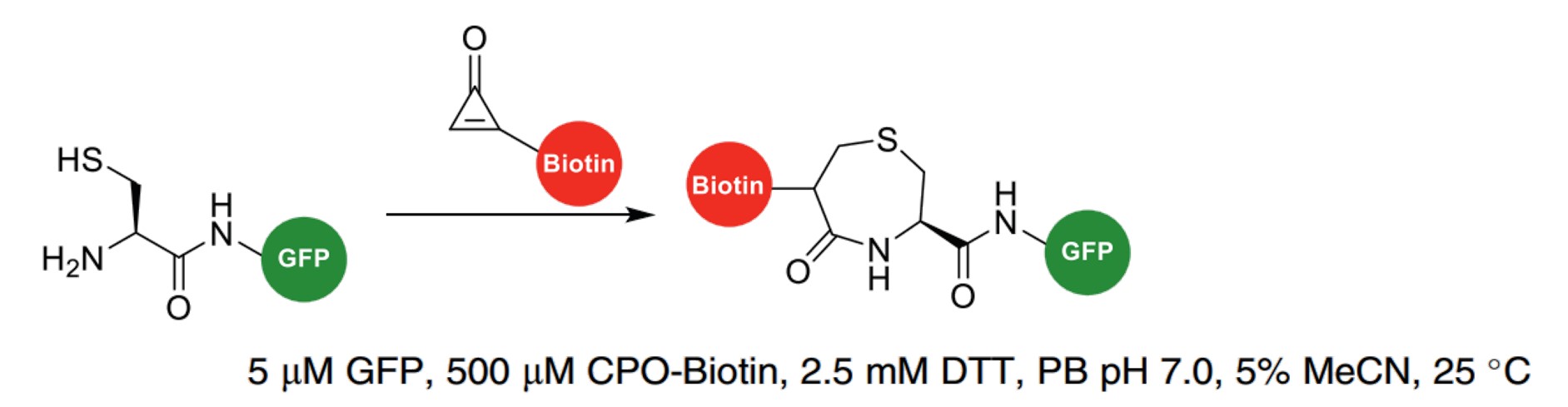
| Cyclopropenone (CPO) |
|
CPO is selective for 1,2-aminothiol groups and can react with NCys to form a 1,4-thiazepan-5-one. |
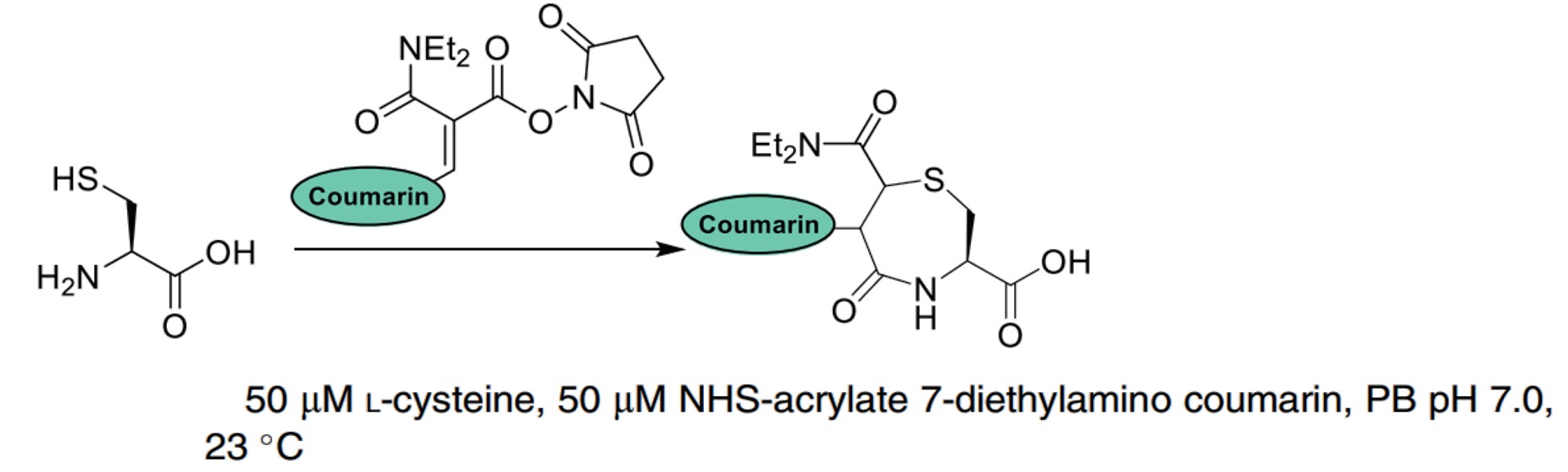
| Reagents containing N-Hydroxy succinimide (NHS) esters and acrylate fragments |
|
These reagents react with NCys to form a 1,4-thiazepan-5-one. |
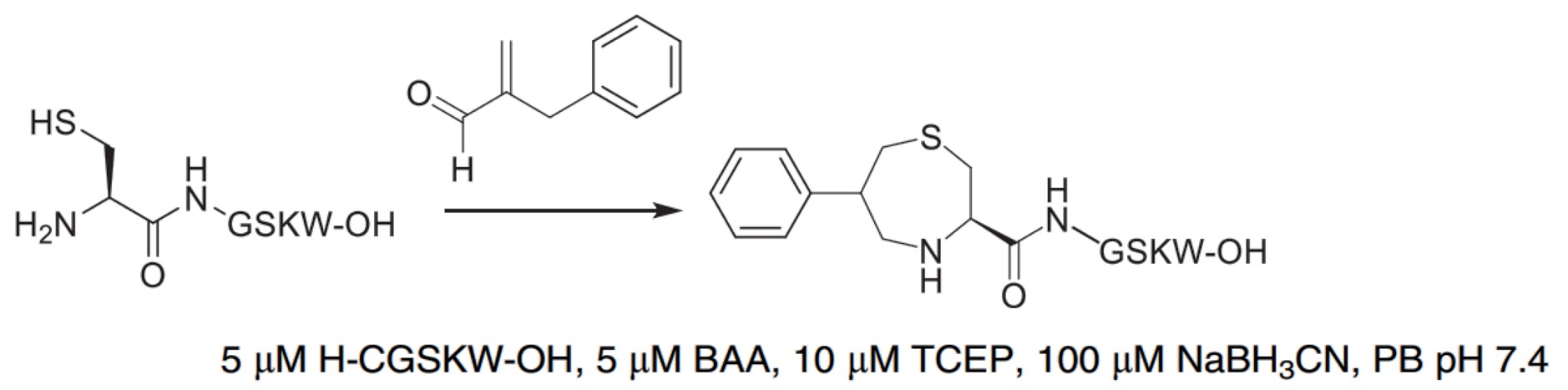
| 2-Benzylacrylaldehyde (BAA) with NaBH3CN |
|
In the presence of NaBH3CN, BBA can react with NCys to form a 1,4-thiazepan-5-one. |
CCys Conjugations
The conjugation methods for CCys primarily include hydrazinolysis and thiophosphonium formation, as outlined in Table 2. These techniques provide efficient pathways for synthesizing CCys derivatives, enabling a range of applications in biochemical research and therapeutic development.
Table 2 Methods for CCys bioconjugation.1, 2
| Strategies | Reactions and Conditions | Mechanisms |
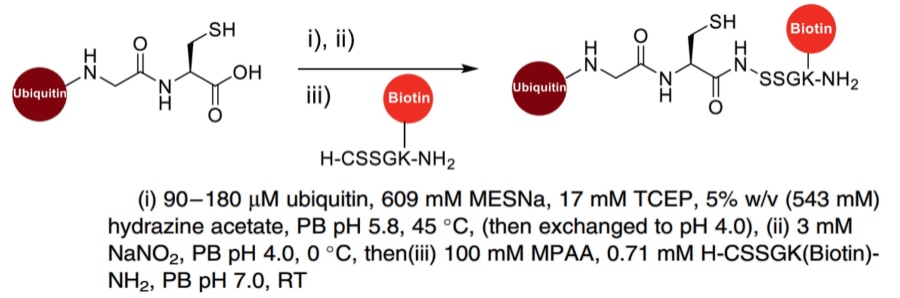
| Hydrazinolysis |
|
The C-terminal protein hydrazide is synthesized through the addition of a hydrazine source, followed by conversion to an acyl azide using sodium nitrite. This intermediate subsequently reacts with sodium 2-mercaptoethanesulfonate (MESNa) to yield a thioester. This resultant thioester can then be efficiently incorporated into NCys-containing peptides via native chemical ligation. |
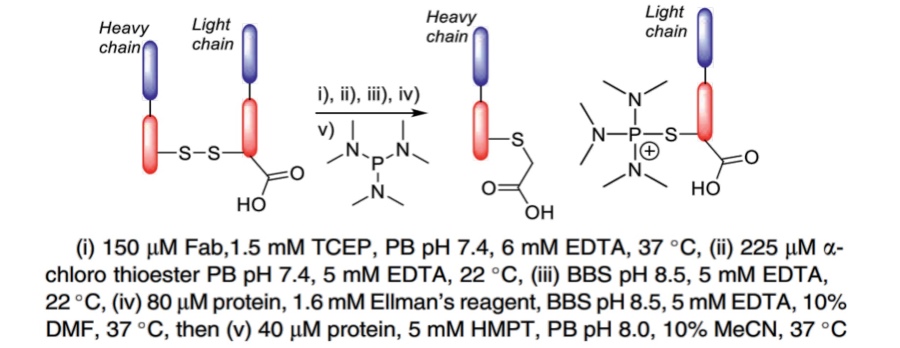
| Thiophosphonium linkage (hexamethylphosphorous triamide (HMPT)) |
|
The unsymmetrical alkyl-aryl (S-SAr) disulfides involving a CCys react with HMPT to form stabilized thiophosphonium adducts. |
Based on professional researchers and proven conjugation technologies, Creative Biolabs can provide site-specific conjugation services for NCys and CCys to meet customer needs. We also offer a variety of crosslinking reagents to assist your research. Please contact us to get more details.
References
- Spears, Richard J., and Vijay Chudasama. "Recent advances in N-and C-terminus cysteine protein bioconjugation." Current Opinion in Chemical Biology 75 (2023): 102306.
- Distributed under open access license CC BY 4.0, with modification.