Lipid- & Peptide-Oligonucleotide Conjugation Therapeutics
Introduction
Oligonucleotide therapeutics present avenues for treating diverse diseases, including genetic disorders, viral infections, and cancer. Oligonucleotides, which are short sequences composed of DNA or RNA, offer the potential to modulate gene expression through several key mechanisms. These mechanisms encompass antisense inhibition, RNA interference (RNAi), and gene silencing. Antisense oligonucleotides function by binding to specific messenger RNA (mRNA) targets, and this binding event effectively prevents the translation of mRNA into protein. Separately, RNA interference (RNAi) harnesses small interfering RNAs (siRNAs) to trigger the degradation of target mRNA, resulting in the selective silencing of the corresponding gene's expression. Gene silencing, in a broader context, encompasses a diverse array of mechanisms through which gene expression can be suppressed, and oligonucleotides may play a crucial role in these intricate processes.
However, the therapeutic potential of oligonucleotides encounters limitations imposed by several factors. These limitations include poor cellular uptake, a challenge that hinders their ability to traverse cell membranes and reach their intended intracellular targets. Furthermore, rapid degradation by nucleases, which are enzymes responsible for breaking down nucleic acids, and unfavorable pharmacokinetic profiles, characterized by swift clearance from the bloodstream and limited tissue distribution, also present significant obstacles. Addressing limitations in oligonucleotide drug delivery and stability, investigations have explored diverse methodologies, with a prominent avenue being the covalent attachment of lipid and peptide moieties to oligonucleotides. This article examines the design, synthesis, and therapeutic applications of lipid- and peptide-oligonucleotide conjugates, highlighting their potential to revolutionize oligonucleotide-based therapies and, perhaps, substantially broaden their clinical utility.
Lipid-Oligonucleotide Conjugates
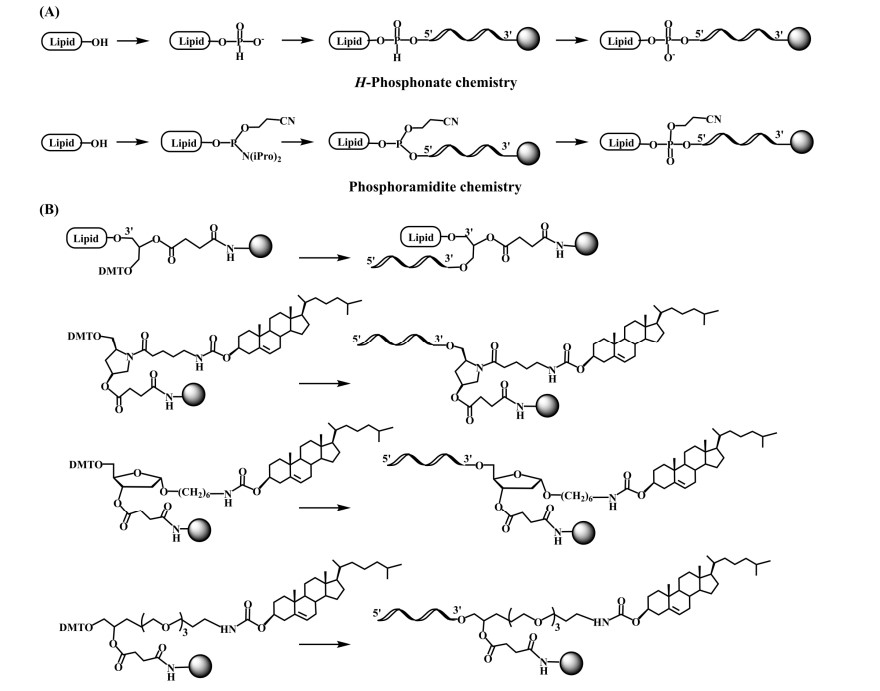 Fig.1 Synthetic approaches for the incorporation of lipids into an oligonucleotide.1
Fig.1 Synthetic approaches for the incorporation of lipids into an oligonucleotide.1
The conjugation of lipid moieties to oligonucleotides has emerged as a pivotal strategy to augment cellular internalization efficiency and optimize pharmacokinetic profiles. This enhancement stems fundamentally from the amphipathic nature of lipids, which enables dynamic interactions with biological membranes through spatially segregated hydrophobic and hydrophilic domains. Three primary mechanisms underlie these improvements:
- Increased Lipophilicity: Lipids increase the lipophilicity of oligonucleotides, facilitating their diffusion across the cell membrane.
- Enhanced Binding to Serum Proteins: Lipids can promote the binding of oligonucleotides to serum proteins, such as albumin, which can act as carriers and prolong their circulation time.
- Formation of Nanoparticles: Lipid conjugation can induce the self-assembly of oligonucleotides into nanoparticles, which can be efficiently taken up by cells through endocytosis.
Lipid conjugation has been applied to various RNA-based therapeutics, including antisense oligonucleotides and siRNAs, to improve their delivery and efficacy.
- Cholesterol Conjugates: Cholesterol was one of the first lipids to be conjugated to oligonucleotides. Cholesterol conjugation has been shown to enhance the cellular uptake and activity of antisense oligonucleotides and siRNAs.
- Fatty Acid Conjugates: Conjugation with fatty acids, such as docosahexaenoic acid (DHA), has also been explored to improve brain delivery of oligonucleotides.
- Lipophilic Vitamin Conjugates: Vitamin E (tocopherol) conjugation has been shown to enhance the delivery of oligonucleotides to the liver.
Peptide-Oligonucleotide Conjugates
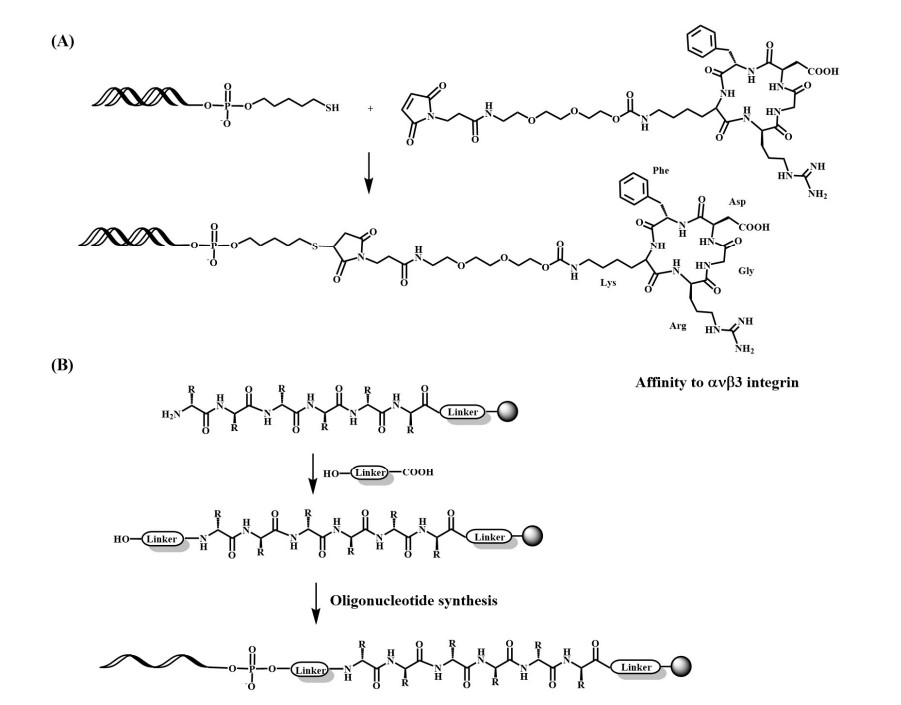 Fig.2 Preparation of oligonucleotide-peptide conjugates.1
Fig.2 Preparation of oligonucleotide-peptide conjugates.1
To overcome oligonucleotide delivery limitations, peptides have emerged as promising alternatives, offering enhanced cellular uptake and cell-type specificity, akin to lipid-DNA conjugates. Peptide moieties facilitate oligonucleotide delivery through mechanisms such as:
- Receptor-Mediated Endocytosis: Peptides can be designed to bind to specific cell surface receptors, triggering receptor-mediated endocytosis and facilitating the internalization of the oligonucleotide conjugate.
- Cell-Penetrating Peptides (CPPs): CPPs are short peptides that can traverse cell membranes and deliver cargo molecules, including oligonucleotides, across cellular barriers.
The significant potential of peptides as delivery ligands, particularly for siRNA, is driving active research into diverse peptide-oligonucleotide conjugation strategies to expand their utility in targeted nucleic acid therapeutics. Key applications include:
- Homing Peptide Conjugates: Developed via phage display technology, homing peptides have been used to deliver antisense oligonucleotides to cardiac tissue, and siRNAs and DNA plasmids to the spinal cord or microglia.
- CPP Conjugates: CPPs (including Arg-rich peptides, antennapedia, transportan, C105Y, etc.) are established tools for enhancing oligonucleotide delivery. Beyond nucleic acid transport, CPP conjugation to peptide nucleic acids (PNAs) is emerging as a promising strategy for developing novel antibacterial agents.
Multifunctional Conjugates
The clinical success of liver-targeting oligonucleotide conjugates has spurred the creation of sophisticated, multi-ligand nucleic acid constructs for therapy. These constructs, which can take the form of single oligonucleotides or DNA nanoassemblies, are engineered with multiple ligands (identical or diverse), including peptides and lipids, to improve therapeutic performance. Current research focuses on optimizing key aspects of oligonucleotide delivery and function, such as target recognition, serum stability, cellular uptake (via receptor targeting or cell-penetrating peptides), and endosomal escape, utilizing strategies like histidine-rich peptides, bombesin peptides, cyclic RGD peptides, and sophisticated conjugation chemistries.
Leveraging extensive experience and a cutting-edge technology platform in oligonucleotide conjugation chemistry, Creative Biolabs offers comprehensive and efficient one-stop conjugation solutions tailored to your specific needs. For detailed information or to request a quotation, please do not hesitate to contact us.
Reference
- Fàbrega, Carme, et al. "Lipid and peptide-oligonucleotide conjugates for therapeutic purposes: from simple hybrids to complex multifunctional assemblies." Pharmaceutics 15.2 (2023): 320. Under open access license CC BY 4.0, without modification.
