AOC Mediated RNA Therapeutics for Muscle Targeting
Introduction
Oligonucleotide therapeutics represent a powerful modality for treating diseases at their genetic source. By designing short strands of nucleic acids, it is possible to modulate RNA function to degrade mRNA, alter splicing, or inhibit translation. While this approach has seen considerable success, its application has been largely confined to the liver. This is because systemically administered oligonucleotides are readily taken up by hepatocytes, but their poor drug-like properties, including rapid clearance and inability to permeate most cell membranes, have limited their delivery to and pharmacological activity in extrahepatic tissues. Antibody-oligonucleotide conjugates (AOCs) have emerged as a promising strategy to address this critical delivery challenge, enabling the targeting of oligonucleotides to tissues beyond the liver, such as skeletal and cardiac muscle.
In this study, scientists investigated the feasibility of using monoclonal antibodies targeting the transferrin receptor 1 (TfR1), which is highly expressed in muscle cells, to deliver various classes of therapeutic oligonucleotides. By creating AOCs, they demonstrated that a single intravenous dose could achieve potent, selective, and durable gene silencing in the skeletal and cardiac muscle of both mice and, significantly, non-human primates. The results showed that this receptor-mediated delivery strategy was substantially more efficient than delivery via non-targeted lipid conjugation (cholesterol) and vastly superior to using unconjugated "naked" oligonucleotides. Furthermore, the study established that the AOC platform is versatile, successfully delivering siRNAs, antisense oligonucleotides (ASOs), and phosphorodiamidate morpholino oligomers (PMOs), confirming its potential as a broad enabling technology for treating a range of muscle diseases.
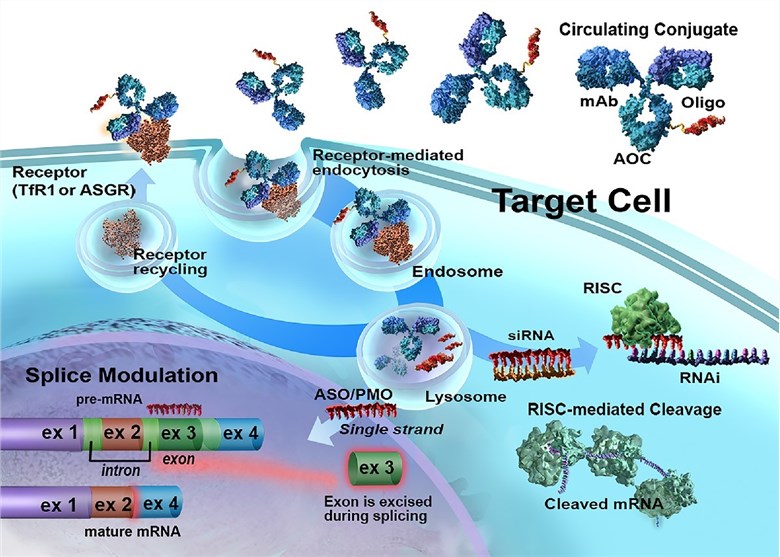 Fig.1 The action mechanism of AOC.1
Fig.1 The action mechanism of AOC.1
Synthesis and Characterization of AOCs
The construction of the AOCs was a multi-step process requiring precise chemical synthesis and bioconjugation. The therapeutic payloads—siRNA, ASO, and PMO—were assembled using standard solid-phase phosphoramidite chemistry, incorporating various chemical modifications (e.g., 2'-fluoro, 2'-OMe, phosphorothioates) to enhance stability and potency. The targeting vehicles were rat anti-mouse TfR1 antibodies for rodent studies and a newly developed mouse anti-human TfR1 antibody (αhTfR1), which is cross-reactive in cynomolgus monkeys, for primate studies. AOCs were then generated using a random cysteine conjugation method, where interchain disulfide bonds on the antibody were partially reduced with TCEP to expose free thiols, which were subsequently reacted with a maleimide linker-oligonucleotide. The resulting conjugates were purified using strong anion exchange or hydrophobic interaction chromatography to achieve a defined drug-to-antibody ratio (DAR) of approximately 1-2.5. Crucially, analysis via ELISA confirmed that the conjugation process had no impact on the antibody's binding affinity to its TfR1 target, ensuring the "guidance system" of the conjugate remained fully functional.
AOC-Mediated Delivery and Activity in Mice
In vivo studies in mice demonstrated the antibody-dependent selectivity of the AOC platform. A siRNA targeting Ctnnb1 conjugated to an anti-TfR1 (skeletal muscle-targeting) antibody resulted in ~9-fold greater siRNA concentration in the gastrocnemius muscle compared to a conjugate using an anti-ASGR (liver-targeting) antibody. Conversely, the anti-ASGR AOC achieved ~6-fold greater concentration in the liver, achieving a 50% effective dose (ED50) comparable to the industry-standard GalNAc-siRNA conjugates. The most significant findings came from muscle-targeting experiments. Anti-TfR1 AOCs produced potent, dose-dependent knockdown (>80%) of multiple gene targets (myostatin (Mstn), myotonic dystrophy protein kinase (Dmpk), small RNA binding exonuclease protection factor La (Ssb)) across a broad panel of skeletal muscles and in the heart. This targeted delivery was at least 10-fold more potent than a non-targeted cholesterol-siRNA conjugate. The pharmacodynamic properties revealed remarkable tissue selectivity; the EC50 for mRNA reduction by a αTfR1-siSsb conjugate was over 75-fold lower in skeletal muscle than in the liver, indicating that receptor-mediated uptake into muscle is a highly productive pathway for pharmacological activity.
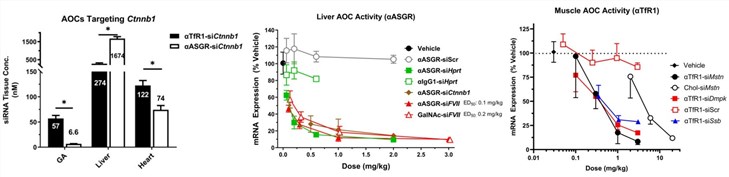 Fig.2 Results of AOC tissue targeting, liver activity, and muscle activity.1
Fig.2 Results of AOC tissue targeting, liver activity, and muscle activity.1
Translation of AOC Delivery and Activity to Non-Human Primates
A critical test for any new therapeutic platform is its translation from rodent models to higher species. To this end, the researchers administered a single 6 mg/kg intravenous dose of an anti-human TfR1 AOC carrying a siRNA against the SSB gene (αhTfR1-siSSB) to cynomolgus monkeys. The results were highly successful and consistent with the mouse data. At 28 days post-dose, the AOC had delivered therapeutically relevant concentrations of the siRNA to various skeletal muscles, including the gastrocnemius and quadriceps. This delivery resulted in a profound and durable pharmacological effect, with up to 75% reduction of the target SSB mRNA. As seen in mice, the activity was highly selective for striated muscle (skeletal and heart), with no meaningful mRNA reduction observed in other major organs like the liver, kidney, or lung. This successful demonstration in a primate model provides strong preclinical validation for the AOC platform's potential utility in humans.
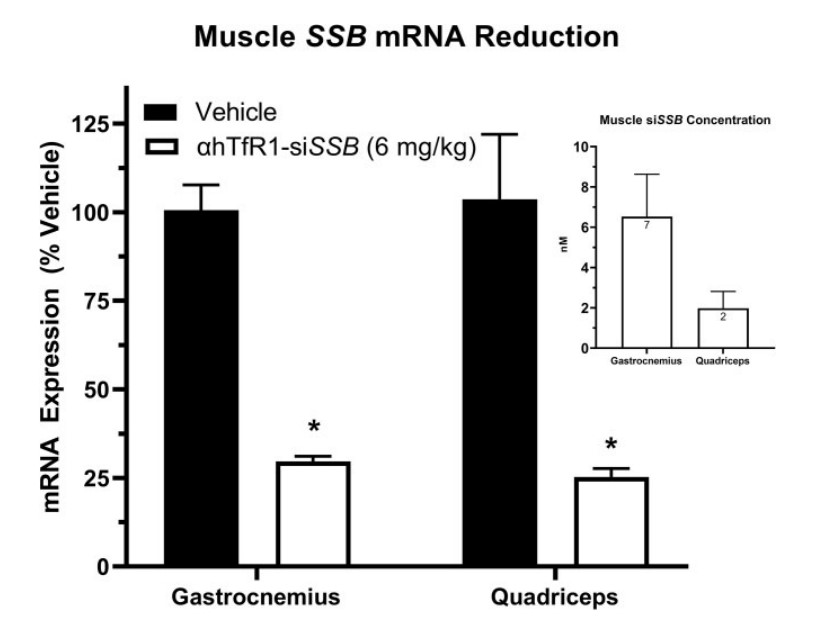 Fig.3 TfR1-siSSB effectively delivers siRNA to muscle, resulting in significant mRNA reduction in cynomolgus monkeys.1
Fig.3 TfR1-siSSB effectively delivers siRNA to muscle, resulting in significant mRNA reduction in cynomolgus monkeys.1
AOCs Versatility Across Oligonucleotide Chemistries
To establish the breadth of the AOCs, their applicability was tested with oligonucleotides that utilize different mechanisms of action. In addition to the double-stranded siRNA, single-stranded ASOs and charge-neutral PMOs were conjugated to the targeting antibodies. An anti-TfR1-ASO conjugate targeting Dmpk produced >75% mRNA knockdown in the skeletal muscle of mice, demonstrating efficacy for ribonuclease H1-mediated mRNA degradation. Likewise, an anti-ASGR-PMO conjugate designed to induce exon skipping of the Pah pre-mRNA was shown to be active in the liver. Interestingly, the study noted that the oligonucleotide chemistry itself influenced biodistribution; the anti-TfR1-ASO conjugate led to significant off-target activity in the liver, whereas the anti-TfR1-siRNA conjugate did not, suggesting that while the antibody dictates primary targeting, the intrinsic properties of the oligonucleotide payload also play a role in the final disposition and activity profile.
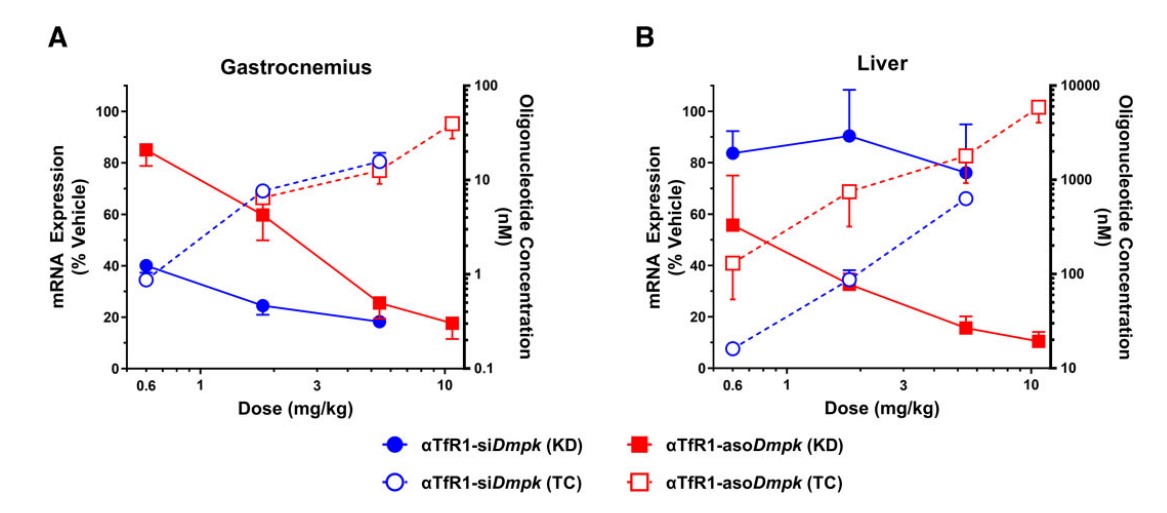 Fig.4 TfR1 AOCs carrying either siRNA or ASO payloads demonstrate strong mRNA silencing activity in muscle.1
Fig.4 TfR1 AOCs carrying either siRNA or ASO payloads demonstrate strong mRNA silencing activity in muscle.1
The research by Malecova et al. provides a compelling, evidence-based demonstration that AOCs can solve the long-standing challenge of delivering RNA therapeutics to muscle tissue. The use of an anti-TfR1 antibody enables potent, durable, and selective gene silencing that is translatable from rodents to non-human primates. This advancement offers a promising avenue for creating innovative therapies for numerous genetic neuromuscular diseases.
At Creative Biolabs, we recognize the immense potential of this technology. We are equipped with the deep scientific expertise required to navigate the complexities of bioconjugation chemistry. Beyond antibody conjugates, we specialize in peptide-oligonucleotide conjugation services, providing our clients with a comprehensive suite of solutions to create precisely targeted therapeutics for any research. Contact us for more information.
Reference
- Malecova, Barbora, et al. "Targeted tissue delivery of RNA therapeutics using antibody–oligonucleotide conjugates (AOCs)." Nucleic Acids Research 51.12 (2023): 5901-5910. Under open access license CC BY 4.0, without modification.
Related Services
- Custom Protein-Oligonucleotide Conjugation Service
- Custom Oligonucleotides-Peptide Conjugation Service
- Custom Oligonucleotides-Bead & Particle Conjugation Service
- Custom Oligonucleotides-Small Molecule Conjugation Service
