Chlorogenic Acid-Targeted Liposomes for Colitis Treatment
Introduction
Inflammatory Bowel Disease (IBD), encompassing conditions like Ulcerative Colitis, represents a significant global health challenge, characterized by chronic inflammation of the gastrointestinal tract. While various therapeutic agents exist, their efficacy is often hampered by issues such as low bioavailability, instability, and lack of targeted delivery to the inflamed colonic tissue, leading to systemic side effects and reduced patient compliance.
Chlorogenic acid (CGA), a natural polyphenol, has demonstrated potent anti-inflammatory, antioxidant, and even anticancer properties. However, its clinical translation is limited by its inherent chemical instability and poor oral bioavailability. This underscores the critical need for advanced drug delivery systems capable of protecting CGA, enhancing its absorption, and ensuring its site-specific action within the colon.
In this study, scientists developed Folic Acid-TPGS-modified CGA Liposomes (FTCLP), an innovative drug delivery system, to enhance the anti-inflammatory potential of CGA for treating colitis. The novelty of this approach lies in the multi-functional nature of the liposomes. They not only shield the delicate CGA molecule from enzymatic degradation and harsh pH conditions within the gastrointestinal tract but also employ a folic acid-mediated targeting mechanism, allowing for more precise delivery of CGA directly to the site of disease.
Preparation and Characterization of FTCLP
FTCLP were synthesized using a modified thin-film hydration method, sonication, and a calcium acetate gradient for drug loading, with soybean lecithin, cholesterol, and FA-TPGS as lipid components. Blank liposomes (FTLP) served as controls. Multiple analytical techniques confirmed successful CGA encapsulation and FTCLP's physicochemical properties. Transmission Electron Microscopy (TEM) showed spherical, well-dispersed liposomes, mostly under 200 nm. Dynamic Light Scattering (DLS) indicated an average particle size of approximately 150.63 ± 0.71 nm, a narrow Polydispersity Index (PDI) of 0.198 ± 0.02, and a slightly positive zeta potential (2.61 ± 0.38 mV), suggesting good stability. High encapsulation efficiency (EE) of 84.85 ± 1.20 and drug loading (DL) of 11.67 ± 0.04 were achieved. Fourier-Transform Infrared Spectroscopy (FTIR) verified the presence of liposomal and folic acid peaks, with CGA's spectral changes confirming its incorporation. Differential Scanning Calorimetry (DSC) revealed the amorphous or molecularly dispersed state of CGA within the liposomes, evidenced by the missing crystalline melting peak of CGA in the FTCLP thermogram.
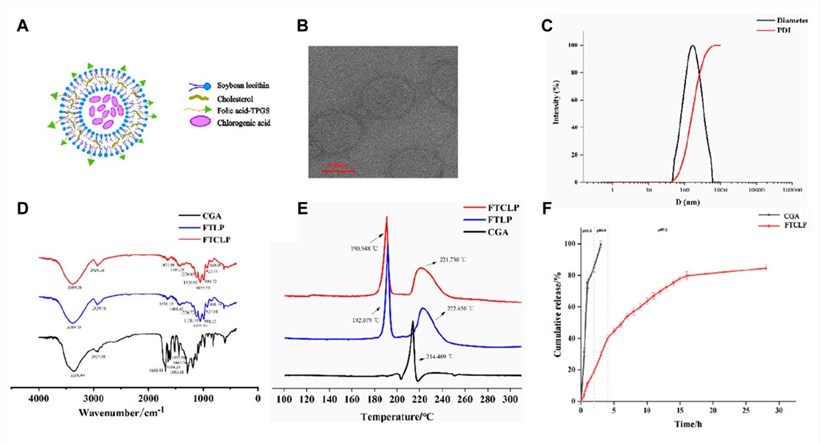 Fig.1 Characterization and in vitro release of FTCLP. (A) The structure of FTCLP, (B) TEM image of FTCLP, (C) Size and PDI of FTCLP, (D) FTIR, (E) DSC, (F) In vitro release of CGA from FTCLP.1
Fig.1 Characterization and in vitro release of FTCLP. (A) The structure of FTCLP, (B) TEM image of FTCLP, (C) Size and PDI of FTCLP, (D) FTIR, (E) DSC, (F) In vitro release of CGA from FTCLP.1
In Vitro Release of CGA from FTCLP
The in vitro release of CGA from FTCLP was assessed via a dialysis bag method, simulating gastrointestinal pH transitions (pH 1.2, 6.8, and 7.4). Free CGA showed rapid release, with nearly 99.70% released within 3 hours. In contrast, FTCLP demonstrated a controlled, sustained release: an initial ~19.03% burst at pH 1.2 was followed by a slower release, reaching ~84.56% cumulatively by 28 hours. This release pattern, fitting the Higuchi model (R2=0.9522), indicated diffusion-controlled kinetics from the liposomal matrix. Such sustained release is advantageous for colonic delivery, potentially prolonging drug exposure and reducing dosing frequency.
In Vivo Efficacy Analysis in a DSS-Induced Colitis Mouse Model
The therapeutic efficacy of FTCLP was evaluated in a well-established dextran sulfate sodium (DSS)-induced colitis model in C57BL/6 male mice. After 7 days of DSS induction to establish colitis, mice were randomly assigned to various treatment groups: a normal control group, a DSS model group (ultrapure water), an FTLP group (blank liposomes, 5 mg/kg BW), a CGA group (free CGA, 5 mg/kg BW), and three FTCLP groups at different doses (FTCLP-L: 2.5 mg/kg BW; FTCLP-M: 5 mg/kg BW; FTCLP-H: 10 mg/kg BW), administered by gavage. Treatment efficacy was assessed through daily monitoring of body weight, stool consistency, and presence of blood (Disease Activity Index), as well as post-mortem analysis of colon length and histological examination (H&E staining).
FTCLP treatment, particularly at medium and high doses, demonstrated superior efficacy in alleviating colitis symptoms compared to both free CGA and blank liposomes. This included significant mitigation of weight loss, a reduction in DAI scores, and a notable restoration of colon length, which is typically shortened during colonic inflammation. Histological analysis revealed that FTCLP treatment reduced epithelial damage, decreased inflammatory cell infiltration, and helped restore normal crypt architecture. Furthermore, FTCLP treatment markedly diminished the levels of pro-inflammatory cytokines, including IFN-γ, IL-1β, and IL-6, in both serum and colon tissue. Gene expression analysis via RT-PCR showed that FTCLP suppressed the colonic mRNA expression of IFN-γ, IL-1β, IL-6, TNF-α, and the key inflammatory transcription factor NF-κB p65, while concomitantly upregulating the expression of JAK and STAT3, suggesting modulation of critical inflammatory signaling pathways.
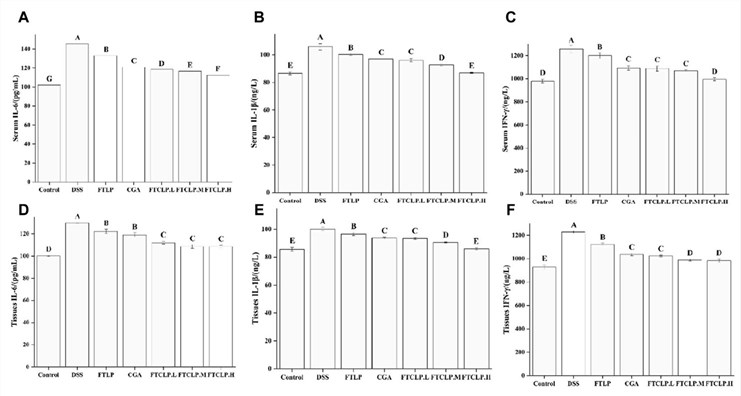 Fig.2 FTCLP decreased the levels of pro-inflammatory cytokines in the serum and colon tissue of mice.1
Fig.2 FTCLP decreased the levels of pro-inflammatory cytokines in the serum and colon tissue of mice.1
Gut Microbiota Modulation by FTCLP
Recognizing the pivotal role of gut microbiota in IBD pathogenesis, the study investigated the effects of FTCLP on the colonic microbial composition using 16S rRNA gene sequencing. DSS induction led to significant gut dysbiosis, characterized by reduced microbial diversity (alpha diversity: Chaos and Shannon indices) and altered community structure (beta diversity: NMDS and PCoA analyses) compared to healthy controls. Treatment with FTCLP, particularly in the medium-dose group, effectively ameliorated these DSS-induced microbial alterations. Specifically, FTCLP administration led to an increase in the relative abundance of potentially beneficial bacteria, such as Lactobacillaceae and Peptostreptococcaceae, while concurrently decreasing the abundance of certain pathogenic or inflammation-associated bacteria, including Bacteroidaceae, Rikenellaceae, and Helicobacteraceae. The Firmicutes/Bacteroidetes (F/B) ratio, often reduced in IBD and indicative of dysbiosis, was significantly restored in the FTCLP-treated group towards the levels observed in healthy controls. Linear discriminant analysis Effect Size (LEfSe) identified specific taxa that were differentially abundant across groups, highlighting the enrichment of Lactobacillales and Lactobacillaceae in the FTCLP group. Correlation analysis further suggested that the observed shifts in gut microbiota composition induced by FTCLP were associated with the attenuation of inflammatory markers, indicating that microbiota modulation is a key component of FTCLP's therapeutic mechanism.
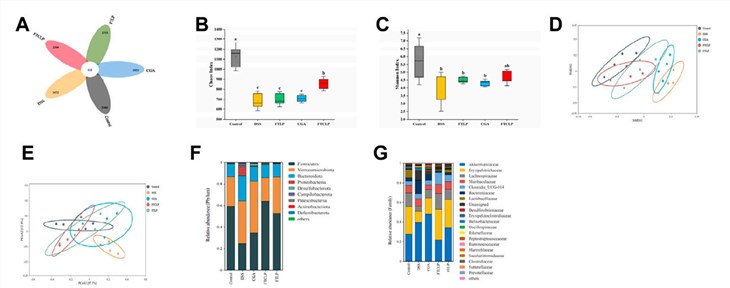 Fig.3 FTCLP regulated the abundance and diversity of gut microbiota. (A) Venn diagram, (B) Chaos index, (C) Shannon index, (D) NMDS analysis at the phylum level, (E) PCoA analysis at the phylum level, (F) phylum-level abundance, (G) family-level abundance.1
Fig.3 FTCLP regulated the abundance and diversity of gut microbiota. (A) Venn diagram, (B) Chaos index, (C) Shannon index, (D) NMDS analysis at the phylum level, (E) PCoA analysis at the phylum level, (F) phylum-level abundance, (G) family-level abundance.1
This study shows that encapsulating chlorogenic acid in FTCLP enhances its stability, sustained release, and anti-inflammatory efficacy in a preclinical colitis model. The formulation mitigated clinical symptoms and colon damage, beneficially modulated inflammatory pathways, and restored gut microbiota. These findings highlight the therapeutic potential of FTCLP as a targeted and effective delivery system for CGA in the management of IBD. The development of such sophisticated, target-modified liposomal systems represents a promising avenue for improving the treatment of inflammatory conditions.
For researchers and pharmaceutical companies looking to harness the power of targeted nanomedicine, Creative Biolabs offers comprehensive development services for creating and characterizing custom target-modified liposomes and other advanced drug delivery systems, helping to translate innovative therapeutic concepts into tangible solutions. Contact us for more details.
Reference
- Li, Qing-qing, et al. "Enhanced anti-inflammatory activity of chlorogenic acid via folic acid-TPGS-modified liposomes encapsulation: characterization and In vivo evaluation on colitis mice." Frontiers in Pharmacology 15 (2024): 1437773. Under open access license CC BY 4.0, without modification.
Related Services
- Custom Small Molecule-Liposome Conjugation Service
- Custom Peptide-Liposome Conjugation Service
- Custom Antibody-Liposome Conjugation Service
- Small Molecule Synthesis and Modification Service
