Glycoproteomics-based Liquid-biopsy LDT Development Service for Nasopharyngeal Carcinoma
Importance of Glycosylation in Nasopharyngeal Carcinoma
Nasopharyngeal cancer (NPC) is a highly metastatic malignancy, particularly prevalent in Southeast Asia and Southern China. While radiotherapy effectively targets early-stage NPC, over 70% of cases are diagnosed at advanced stages. Although Epstein-Barr virus (EBV) DNA and EBV serology tests have been suggested for early diagnosis and screening of NPC, their sensitivity varies, and the molecular mechanisms driving the progression of NPC remain unclear. Consequently, there is an urgent need for accurate and reliable methods for the early diagnosis and assessment of NPC. Aberrant protein glycosylation plays a crucial role in various neoplastic diseases and may be linked to different stages of cancer. Thus, glycoproteomic technology holds great promise in overcoming previous limitations and has the potential to identify more precise glycoprotein biomarkers for the early diagnosis, evaluation, or prognosis of NPC.
Glycoproteomics-based Liquid-biopsy LDT Development for NPC at Creative Biolabs
Creative Biolabs has successfully established a specialized and comprehensive laboratory-developed test (LDT) platform by leveraging our expertise in glycoproteomics and state-of-the-art technology. Our LDT development offers one-stop services for large-scale glycoproteomics analysis, encompassing every step from sample preparation to high-throughput, high-sensitivity MS-based detection, and culminating in in-depth bioinformatics analysis. Overall, based on our LDT platform, we provide not only multi-dimensional insights for glycosylation patterns, protein functions, and disease pathways but also diagnosis biomarkers to differentiate the stages of disease progression in NPC.
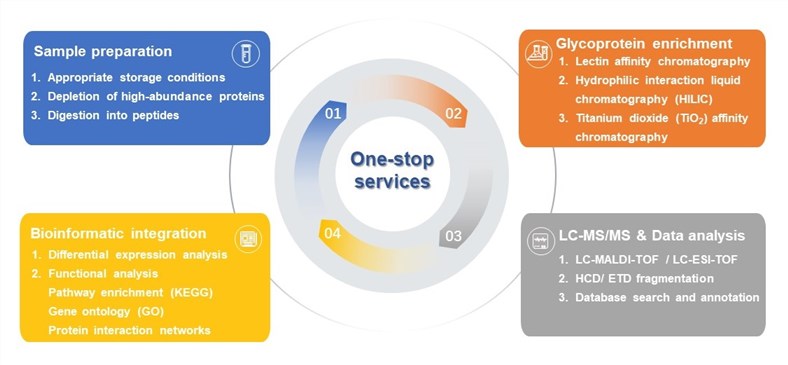 Fig.1 One-stop services of glycoproteomics-based LDT development for NPC.
Fig.1 One-stop services of glycoproteomics-based LDT development for NPC.
LDT Development Highlights at Creative Biolabs

Advanced database search
Inclusively covering most of the reported N-glycan structures and O-glycan structures.

Comprehensive Information
Offering complete details encompassing glycopeptides, glycosylation modification sites, and the entire glycan composition and structures.

High-quality control of MS data
Quality controls like assessing peptide length distribution, parent ion mass tolerance distribution, peptide count distribution, and protein coverage distribution are performed to ensure that the results meet the established standards.

In-depth analysis of multi-omics
Delving into information by differential expression analysis, clustering analysis, functional analysis, and protein interaction networks to explore novel mechanisms and extract deeper insights.

Rapid data retrieval
Utilizing a distinctive retrieval method, which compresses the previously weeks-long retrieval time to a matter of days, significantly shortening your experimental cycle.
Creative Biolabs is a pioneering leader in the field of glycoproteomic analysis. Our liquid biopsy LDT services based on glycoproteomics offer a highly promising avenue to enhance the early diagnosis and prognosis of NPC. For more detailed information, please feel free to contact us or directly send us an inquiry.
For Research Use Only.
Related Services

 Fig.1 One-stop services of glycoproteomics-based LDT development for NPC.
Fig.1 One-stop services of glycoproteomics-based LDT development for NPC.






