Imaging Mass Spectrometry
Overview of Imaging Mass Spectrometry in Glycan
Specific application of N-glycan targeted MALDI-IMS was reported initially in fresh frozen tissues in 2013, followed a year later in formalin-fixed paraffin-embedded (FFPE) tissue sections. The key to the approach is to spray a molecular coating of peptide N-glycosidase F (PNGase F) on the tissue, an enzyme that cleaves the N-linked glycan structures at their site of attachment to an asparagine residue on the glycoprotein carrier. The released N-glycans are detected in the mass spectrometer after application of an organic acid matrix and ionization by laser desorption, using a raster grid of locations generally 40 to 100 mm apart across the entire tissue. A two-dimensional distribution map of the peak intensities of each released glycan is generated by analysis of the cumulative spectra obtained from each spot on the tissue. The use of a solvent sprayer has allowed robust and reproducible sample preparatory workflows to evolve that facilitate large-scale analysis of clinical tissue cohorts. This basic workflow has been used by multiple laboratories to characterize the N-glycan distributions for multiple cancer types, including breast, prostate, liver, pancreas, ovary, colon, sarcoma, kidney, and lung, as well as in cardiac and other tissue types.
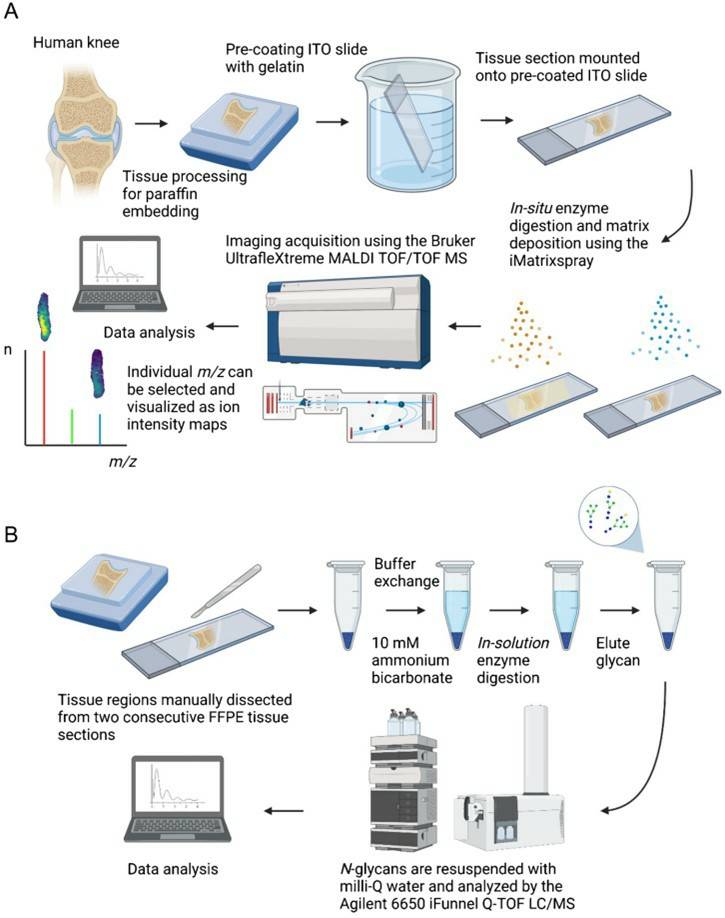 Fig.1 MALDI-MSI and LC-ESI-MS/MS technology-based N-glycan analysis process.1, 3
Fig.1 MALDI-MSI and LC-ESI-MS/MS technology-based N-glycan analysis process.1, 3
Basic Workflow of Imaging Mass Spectrometry
For analytical considerations using the basic workflow, the released glycans are detected directly from the tissue without the need for enrichment or derivatization. The glycans detected depend entirely on the activity of the PNGase F, thus any background signal caused by tissue is minimal. The use of the automated solvent sprayer to apply a reproducible thin molecular coating of PNGase F ensures minimal diffusion and excellent colocalization of glycans to their sites of origin. In addition, because of the sequential nature of their biosynthesis, the basic structural compositions of the N-glycans that can arise are known and well documented. For MALDI-IMS, this results in highly reproducible detection of a core number of ~40 N-glycan species in normal and tumor tissues that can be used for basic biostatistical comparisons across conditions. These glycans also serve as the scaffolds for disease-associated increases in sialic acid and fucose modifications resulting from the expression of different genes in the fucosyltransferase and sialyltransferase families. Some tumor types also are characterized by increases in glycans with bisecting N-acetylglucosamine structures and poly-lactosamine extensions.
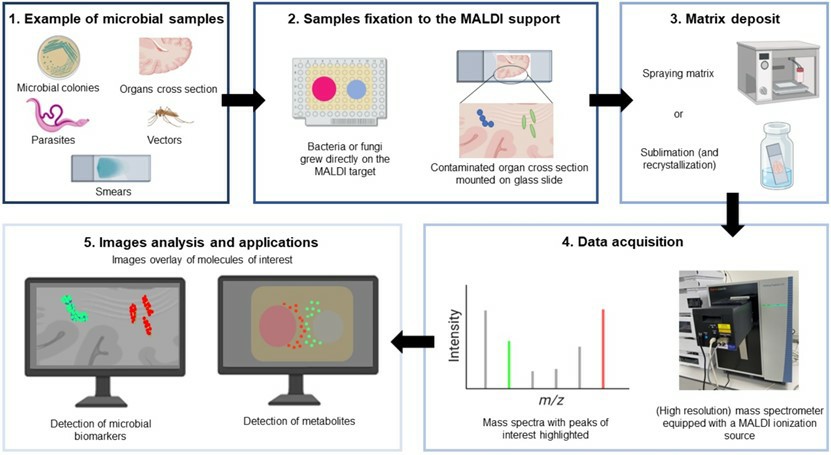 Fig.2 MALDI-IMS workflow.2, 3
Fig.2 MALDI-IMS workflow.2, 3
Advantages of Imaging Mass Spectrometry in Glycan Research
Based on thousands of analyses of individual FFPE tumor tissue slides and tumor cores in tissue microarray slides, several advantages to N-glycan MALDI-IMS are evident relative to other MALDI-IMS targets. A major advantage with regard to clinical translation is the ability to perform these analyses on FFPE tissues, thereby leveraging the predominant specimen type found in clinical histopathology laboratories and tissue biorepositories. The MALDI-IMS workflow fits seamlessly into the pathology laboratory because it uses the same tissue sections required for pathologic evaluation without additional patient interactions. Similarly, the processing steps for the method before PNGase F application are essentially the same as standard histology practices for tissue staining and immunohistochemistry. Because the N-glycans lack free amine groups and are thus not cross-linked by formalin, it has been shown that only dewaxing, but not antigen retrieval, can be used effectively to obtain glycan signals.
Services at Creative Biolabs
From the previous reports, we have known that imaging mass spectrometry has an irreplaceable status in glycan research. As a mature glycoprotein-relevant services provider, Creative Biolabs has accumulated a lot of experience in the application of imaging mass spectrometry in glycan research. With advanced equipment, start-of-art technologies, and an experienced expert team, we are capable of providing high-quality imaging mass spectrometry services to global clients.
If you are interested in our services or you meet any questions during glycoprotein research, please don't hesitate to contact us for more information.
References
-
Lee, Yea-Rin, et al. "Mass spectrometry imaging spatially identifies complex-type N-glycans as putative cartilage degradation markers in human knee osteoarthritis tissue." Analytical and Bioanalytical Chemistry 414.26 (2022): 7597-7607.
-
Feucherolles, Maureen, and Gilles Frache. "MALDI mass spectrometry imaging: a potential game-changer in a modern microbiology." Cells 11.23 (2022): 3900.
-
Distributed under Open Access license CC BY 4.0, without modification.
For Research Use Only.
Related Services

 Fig.1 MALDI-MSI and LC-ESI-MS/MS technology-based N-glycan analysis process.1, 3
Fig.1 MALDI-MSI and LC-ESI-MS/MS technology-based N-glycan analysis process.1, 3
 Fig.2 MALDI-IMS workflow.2, 3
Fig.2 MALDI-IMS workflow.2, 3

