Native Chemical Ligation for Glycoprotein Synthesis Service
Native chemical ligation (NCL) is a useful method for synthetic and semi-synthetic proteins synthesis. It is excited that a synthetic component in NCL can be site-specifically glycosylated, retaining native sugar-protein linkages. This technology allows the assembly of glycoproteins that are not readily available from biological sources. With extensive experience in employing NCL for glycoprotein synthesis, scientists of Creative Biolabs are proficient in synthesizing glycoprotein up to 150 amino acids in length.
The Background of Glycoprotein Synthesis
Glycoproteins are featured by a covalent linkage between carbohydrates through asparagine and serine or threonine. The carbohydrates are usually N-acetyl glucosamine, N-acetyl galactosamine, mannose, xylose, and fucose. Sufficient quantities of homogeneous glycoproteins are often not available from biological sources to facilitate glycoprotein research. Previous studies have shown that the total chemical synthesis of certain glycopeptide components of glycoproteins is possible. Indeed, the difficulty associated with glycoprotein synthesis has precluded much biological research using entirely chemical approaches.
Applications of NCL
In the process of NCL, a C-terminal peptide thioester reacts with an N-terminal cysteinyl peptide to produce a native peptide bond between the two fragments, which is convenient to use NCL for the synthesis of active proteins from smaller fragments. Such an approach allows protein modifications (including glycosylation, phosphorylation, acetylation, methylation, and sulfation) to be introduced in a controlled fashion into smaller peptide fragments that are amenable to total chemical synthesis. With defined sequence and structure, these fragments can be ligated into full-length proteins. NCL allows controlling the identity and the position of glycosylation, which is only limited by the imagination and capability of the synthetic chemist, making NCL the most popular chemical approach for glycoprotein synthesis.
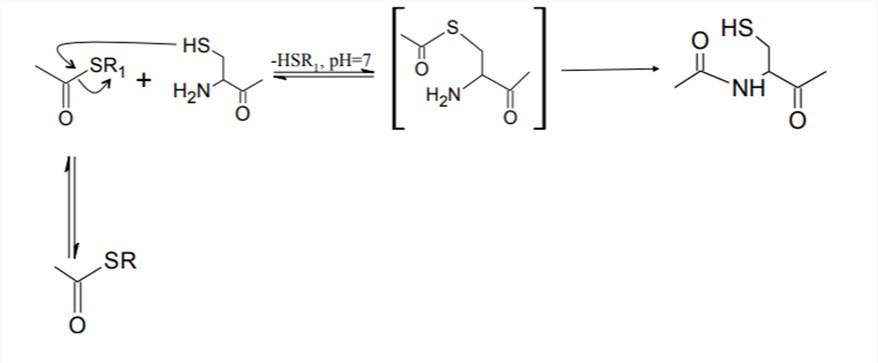 Fig.1 The mechanism of NCL.1
Fig.1 The mechanism of NCL.1
NCL for Glycoprotein Synthesis Service at Creative Biolabs
It is widely accepted that approximately 50 amino acid residues represent the limit for efficient automated synthesis and the difficulties are associated with glycoprotein synthesis. To address this, Creative Biolabs has introduced a group of scientists who have a distinguished understanding of glycoprotein synthesis. NCL has greatly extended the size of proteins that can be synthesized. Based on NCL, we have successfully synthesized glycoproteins up to 150 amino acids in length. We are fully competent and dedicated to serving as your one-stop-shop for glycoprotein synthesis and characterization.
Highlights
-
NCL for glycoprotein synthesis
-
Total chemical synthesis
-
Synthesis of glycoprotein up to 150 amino acids in length
-
Highly professional Ph.D. level scientists
Equipped with professional scientific staff and world-leading technology platforms in glycoprotein research, Creative Biolabs is pleased to share our cutting-edge technology and extensive expertise in using NCL for glycoprotein synthesis. If you are interested in glycoprotein synthesis, please contact us for more information and a detailed quote.
Published data
As a key tool in the field of biochemical synthesis, NCL is widely applied in the synthesis of proteins and peptides. It can connect two polypeptide fragments in an efficient and substrate-specific manner, which not only retains the original peptide backbone structure but also realizes the precise control synthesis of the polypeptide chain, which is of great significance for maintaining the biological activity of the peptide. As shown in the figure below, when free cysteine and thioester functional groups exist in the same peptide fragment, an intramolecular nucleophilic reaction will occur to form a cyclic peptide connected by a thioester bond, and then the final cyclic molecule connected by a natural peptide bond is generated through SN acyl transfer. These cyclic proteins can be synthesized quickly and efficiently using the NCL reaction. The NCL reaction plays a key role in biomedical research and drug development, promotes the study of protein structure and functions, and helps to design and synthesize new drug molecules. The development of this method has promoted progress in protein engineering, drug design, and biotechnology.
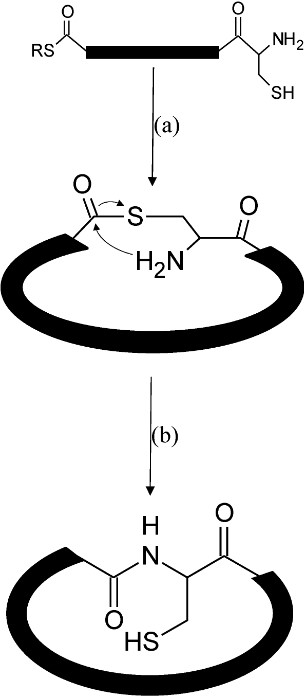 Fig.2 Synthesis mechanism of cyclic peptides.1
Fig.2 Synthesis mechanism of cyclic peptides.1
FAQs
Q1: What types of carbohydrate moieties can be incorporated into glycoproteins using NCL?
A1: NCL allows the incorporation of various types of carbohydrates such as N-acetyl glucosamine, N-acetyl galactosamine, mannose, xylose, fucose, etc. These carbohydrates can be site-specifically attached to asparagine, serine, or threonine residues within the peptide sequence.
Q2: What are the advantages of using NCL over biological methods for glycoprotein synthesis?
A2: The primary advantages include the ability to achieve homogeneity and precise control over the site and type of glycosylation, overcoming the heterogeneity often associated with glycoproteins obtained from biological sources. Additionally, NCL allows for the assembly of glycoproteins that may be difficult or impossible to express in biological systems.
Q3: Can you synthesize glycoproteins with multiple glycosylation sites?
A3: Yes, Creative Biolabs synthesizes glycoproteins with multiple glycosylation sites. The NCL method allows for precise control over the number and position of glycosylation sites, enabling the synthesis of complex glycoproteins with multiple carbohydrate modifications.
Customer Review
Efficient Glycoprotein Synthesis
"The Creative Biolabs team successfully synthesized the glycoprotein we needed using NCL technology. The whole process was efficient and accurate, and the glycosylation position was precise, providing key experimental materials for our project."
Flexible Customization Service
"We are particularly grateful to Creative Biolabs for its flexible customization service on glycosylation modification. They were able to synthesize glycoproteins with specific modifications using NCL technology precisely according to our needs, which greatly advanced our research progress."
References
-
Thapa, Parashar, et al. "Native chemical ligation: a boon to peptide chemistry." Molecules 19.9 (2014): 14461-14483. Distributed under Open Access license CC BY 3.0, without modification.
For Research Use Only.
Related Services

 Fig.1 The mechanism of NCL.1
Fig.1 The mechanism of NCL.1
 Fig.2 Synthesis mechanism of cyclic peptides.1
Fig.2 Synthesis mechanism of cyclic peptides.1

