Staudinger Ligation for Glycoprotein Synthesis Service
With abundant knowledge in glycoscience and dedicated commitment to the scientific community, Creative Biolabs is pleased to share our knowledge and passion in employing Staudinger ligation for homogenous glycoprotein synthesis to facilitate our customers’ research and project development.
Background of Glycoprotein Synthesis
As the most complex post-translational modification of proteins, glycosylation plays extremely important roles in modulating protein structure and localization, cell-cell recognition and signal transduction. However, the isolation of homogeneous glycoproteins from natural sources in significant quantity remains a challenge. Chemical synthesis of glycoproteins can provide well-defined materials to serve as tools for the investigation of their structures and biological properties. Synthetic glycoproteins allow the introduction of unnatural arbohydrate moieties for additional glycoprotein studies and therapeutic development.
Introduction of Staudinger Ligation
Among the methods that have been developed for the preparation of homogenous glycoproteins, convergent glycopeptide coupling represents a promising cost-effective approach. The cysteine-dependent native chemical ligation (NCL) approach has been widely used in glycoprotein synthesis. Synthetic glycopeptide thioesters can be coupled with bulky proteins containing an N-terminal cysteine. Sizable intein-generated protein thioesters can also be coupled with synthetic glycopeptides that contain an N-terminal cysteine. Despite the success of NCL, a Cys at the ligation site is commonly required. However, not all the glycoprotein targets contain a Cys, or the location of Cys might not be appropriate for ligation. An interesting alternative approach to overcoming the limitation of Cys is the "traceless" Staudinger ligation method. In the Staudinger ligation, an azide and phosphinothioester react to form an amide that does not contain any residual atoms from the phosphine prosthetic group. Studies have shown that this ligation is compatible with the unprotected functional groups of proteinogenic amino acids and this ligation occurs in high yields at room temperature in aqueous or wet organic solvents. So far, Staudinger ligation has been applied in diverse fields such as protein immobilization, solid-phase peptide synthesis, stereoselective N-glycosylation, and the synthesis of homogenous glycoproteins.
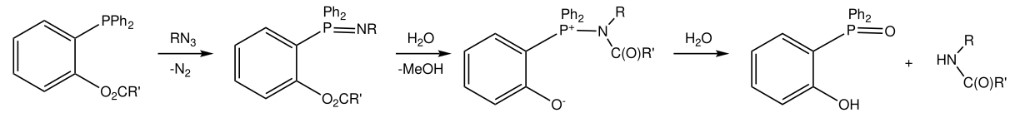 Fig.1 Traceless Staudinger ligation reaction.1
Fig.1 Traceless Staudinger ligation reaction.1
Staudinger Ligation for Glycoprotein Synthesis at Creative Biolabs
With Ph.D. level scientists and extensive experience in glycoscience, Creative Biolabs has perfected our technical pipelines by introducing Staudinger ligation for glycoprotein synthesis. An efficient glycopeptide-coupling reaction under mild conditions, which are compatible with the delicate functional groups in glycoproteins, is employed for homogenous glycoprotein synthesis.
Highlights
-
Under mild conditions that are compatible with the delicate functional groups in glycoproteins
-
High yields
-
Top-rated customer experience
-
Tailored research & services
With extensive experience in glycoscience, Creative Biolabs is an ideal company to entrust with your business in glycoprotein synthesis. We offer turn-key or ala carte services customized to our clients’ needs. Please contact us for more information and a detailed quote.
Reference
-
Image retrieved from https://en.wikipedia.org/wiki/File:Traceless_StaudingerLigation.svg, Smokefoot, 2022, used under CC BY-SA 4.0, without any modification.
For Research Use Only.
Related Services

 Fig.1 Traceless Staudinger ligation reaction.1
Fig.1 Traceless Staudinger ligation reaction.1

