All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
Chimeric antigen receptor (CAR) engineered T cells have demonstrated effective efficacy in the treatment of refractory hematological malignancies. CAR-T technology has become more and more mature and emerges as an important treatment option for various tumor indications. However, one of the challenges associated with CAR-T cell therapy is cytokine release syndrome (CRS).
To address the side effects of CAR-T therapy, Creative Biolabs has developed an innovative modular CAR-(modCAR) in combination with its adaptor molecule (CAR-adapter) that aims to further precisely direct CAR to target cells, thereby addressing a major obstacle to this emerging therapeutic class.
modCAR & CAR-adaptor Design
The universal or modCAR chimeric receptor we developed does not directly recognize the target antigen but instead contacts the adaptor molecule (CAR-adapter), which in turn binds to the target antigen. modCAR-T cells connect with CAR-adapters to form immune synapses, thereby exerting anti-tumor effects.
The implementation of this mechanism strictly depends on the existence of CAR-adaptor. The CAR adaptor has high affinity for tumor antigen and the ECD of modCAR. Our professional cell therapy team can help our customers choose the appropriate CAR adaptor (depending on the half-life, biodistribution, and target antigen) to strictly regulate and adapt the activity of modCAR-T cells.
Currently, a variety of CAR-adaptor can be used to regulate modCAR, including but not limited to:
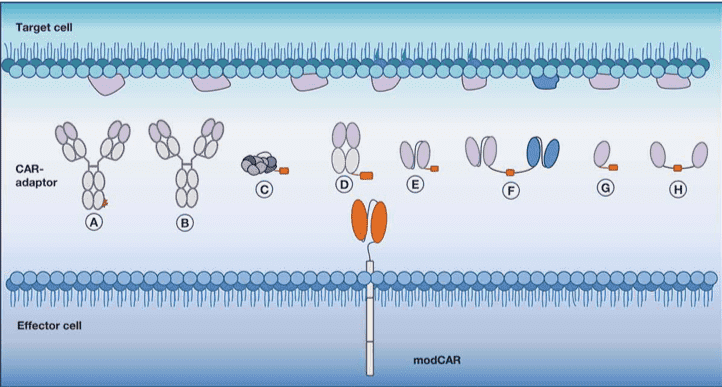
Fig.1 Schematic representation of modCAR & CAR-adaptor.
modCAR-T Combined with Antibodies Adaptors Already Used in The Clinic!
Some modCAR methods use clinically approved therapeutic IgG. Currently, Unum Therapeutics Inc. is evaluating FcγRIIImodCAR-T cells in combination with rituximab for relapsed and refractory B-cell lymphoma (ClinicalTrials.gov identifiers: NCT03189836, NCT02776813). To date, no serious adverse events related to modCAR-T cells have not been observed.
| NCT ID | Program | Conditions | Lead Sponsor | Phase | Update Time |
| NCT03189836 | Study of ACTR707 in Combination With Rituximab in Subjects With Relapsed or Refractory B Cell Lymphoma | Lymphoma | Unum Therapeutics Inc. | Phase 1 | November 19, 2019 |
| NCT02776813 | Study of ACTR087 in Subjects With Relapsed or Refractory B-cell Lymphoma | Lymphoma | Unum Therapeutics Inc. | Phase 1 | November 19, 2019 |
One-stop modCAR Development Services
With state-of-art modCAR development platforms and advanced technologies, Creative Biolabs is capable of offering CAR-T-cell development services. For the regular or custom CAR backbone construction, please refer to our related services: CAR Design & Construction. To further assess your modCAR biological efficacy (e.g., cytokine production, tumor killing, and CAR-T cell proliferation), our scientists can also provide comprehensive downstream services to complete your whole research. Please click CAR Cell In Vitro Assay Service, CAR-T Preclinical In Vivo Assay for more details.
For more detailed information, please feel free to contact us or directly sent us an inquiry.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION