Antiretroviral therapy (ART) has become a stepping stone in the treatment of AIDS, suppressing HIV to an undetectable level in the blood, but it cannot completely eradicate HIV. Instead, the HIV reservoir will remain in the latently infected cells, resulting in a viral rebound following ART interruption, which poses a major challenge for curing HIV infection. In addition, lifetime antiretroviral therapy is expensive and inconvenient, with strong drug toxicity. Therefore, alternative treatment options are highly desirable to maintain viral remission without the use of ART.
Anti-HIV chimeric antigen receptor (CAR) T cells can provide a cure by recognizing and killing Env-specific virus-producing cells. CAR-T cells are T cells genetically engineered to express CAR, which directs or redirects T cells to specifically target the target antigen. CAR usually contains four different domains: extracellular antigen binding domain, hinge, transmembrane domain, and intracellular signaling domain. This allows CAR-T cells to be activated in an MHC-independent manner and makes them less sensitive to common immune escape mechanisms, including the down-regulation of MHC class I molecules.
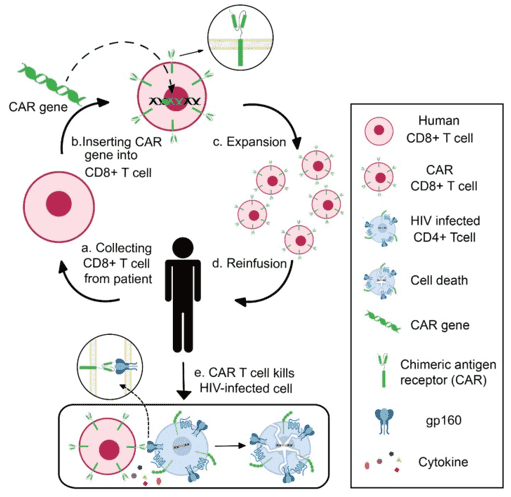 Fig.1 Schematics of CAR T-cell therapy for HIV infection.1
Fig.1 Schematics of CAR T-cell therapy for HIV infection.1
At Creative Biolabs, we have a thorough knowledge of the important aspects of the development of anti-HIV CAR-T cells, with a special focus on the evolution of HIV CAR design to enhance efficacy and target specificity. We are committed to providing the world's leading HIV CAR therapy development services, including HIV CAR vector design, HIV CAR cell design and characterization, preclinical investigation and clinical stage development.
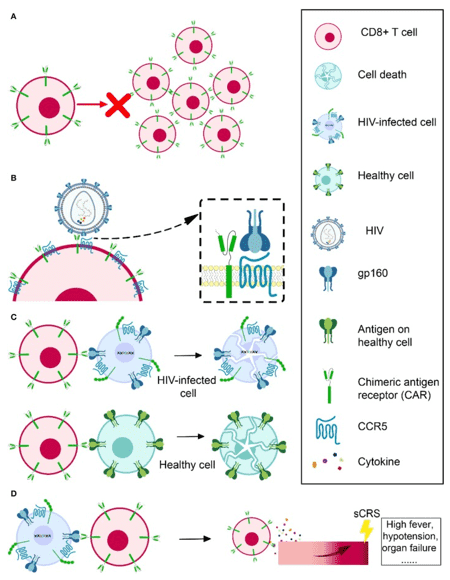 Fig.2 Obstacles in HIV-specific CAR T therapy development.1
Fig.2 Obstacles in HIV-specific CAR T therapy development.1
Despite the above progress, there are still some obstacles to overcome in the process of developing anti-HIV CAR-T cells as a treatment for HIV. Obstacles in HIV-specific CAR-T therapy development mainly refer to:
A. cell expansion and persistence in vivo
B. susceptibility to HIV infection
C. off-target effects
D. severe cytokine release syndrome.
These major obstacles are listed in Figure 2 below.
Creative Biolabs is committed to solving these difficult challenges and providing best-suit research tools for our customers. We provide high-quality customized services covering the entire HIV CAR-T treatment development process to best suit your technology, program, and budget requirements, thus greatly helping you solve these challenges.
As a leading cell therapy biotechnology that provides cell therapy related services, Creative Biolabs masters the most advanced CAR/TCR technology. With the state-of-art CAR development platform, Creative Biolabs is capable of offering a broad range of HIV CAR early development services. Our one-stop HIV CAR therapy development services include but not limited to:
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION