Creative Biolabs is committed to providing canine CAR-T characterization validation services to best suit the client's program requirements.
Preclinical studies reveal the promise and challenges of CAR-T development and therapy. CARTs preclinical evaluation, including a range of responses, such as CARTs activation and exhaustion, tumor killing, and cytokine production, is crucial to the development and optimization of CAR-T cell therapies. These comprehensive evaluation tests usually need to be carried out through multiple dimensions and need to take into account multiple influencing factors. For example, the selection of experimental types, the selection of tumor cells in vitro, the establishment of experimental models in vivo, and so on.
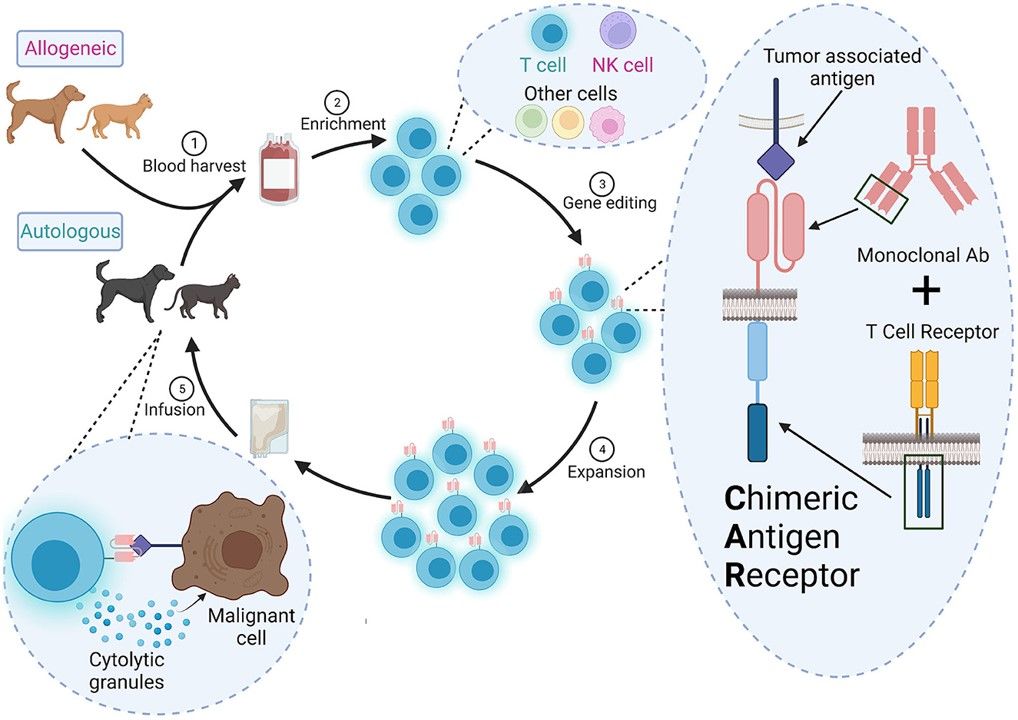 Fig.1 Overall scheme for CAR therapy in veterinary medicine.1
Fig.1 Overall scheme for CAR therapy in veterinary medicine.1
Leveraged by advanced technology platforms and years of experience, Creative Biolabs has launched a full range of evaluation analysis services for global customers to facilitate canine CAR-T research. Our services are featured with the following assays. In addition to these assays, we also provide customized development experiments according to customers' project needs.
Q1. For canine CAR-T development, how does Creative Biolabs select target tumor cells for different targets?
A: In the development, we will first communicate with the customer whether there are relevant reference data or literature. If the target is really rare and appropriate tumor cells cannot be found, we will construct overexpressed cell lines to meet the experimental requirements.
Q2. How does Creative Biolabs secure the source of cells for canine CAR-T development?
A: We strictly abide by the related requirements. PBMC samples collected from blood donors on the day of product collection are tested following related requirements for blood transfusion.
If there is any query, please contact us for more details about the canine CAR-T cell characterization service.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION