With the FDA approval of two CAR-T cell-based therapies, chimeric antigen receptor (CAR)-engineered T cell therapy becomes more and more popular in academic and clinical application. Based on the outstanding expertise and rich experience, Creative Biolabs has specially developed a comprehensive platform for CAR-T cell-based drug discovery. This platform enables us to offer GMP-level plasmid preparation, virus packaging, and T cell production services to help you discover new therapies.
GMP-level Plasmid Manufacture
Cell therapy brings the expectation of overcoming cancer. The first important issue is to produce adequate plasmids for the following virus packing and cell transfection. However, traditional flask culture of Escherichia coli (E. coli) can only be used for small quantity preparation and fails to meet the large-scale needs. To break this limitation, Creative Biolabs utilizes bioreactors for the plasmid manufacture. Aided by our optimized culture medium, production process, and purification kit, we are capable of producing plasmid with hundred milligrams per liter in short 30 hours. In addition, multiple quality control assays can be provided to meet your specific requirement.
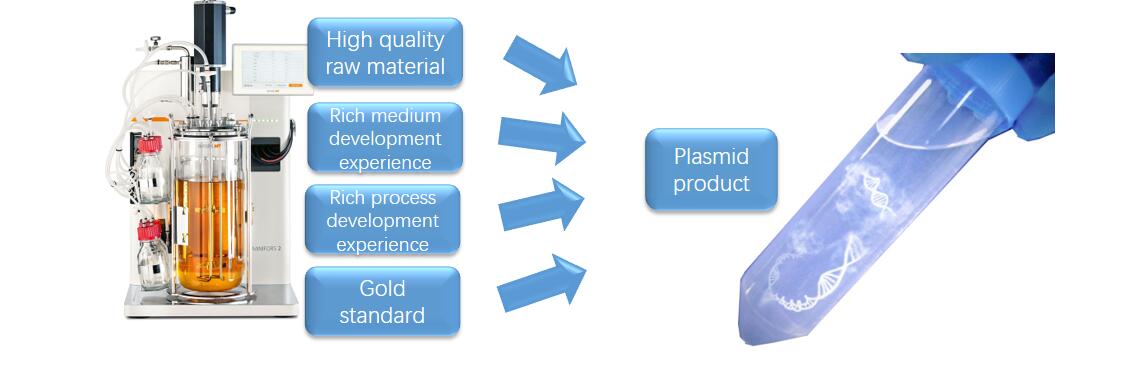
GMP-level Virus Packaging
Virus plays an irreplaceable role in gene transfection. However, the title affects the transfection efficiency directly. Besides, it is challenging for researchers to amplify the packaging process with a controllable, stable and easy manner. After years of efforts, scientists from Creative Biolabs worked out a unique HEK cell line, which showed excellent lentivirus packaging efficacy with our special transfection agent. Both adherent and suspension culture can be applied. We have the capacity to produce large amount of unpurified virus for more than 800 patients and purified high titer virus for more than 50 patients at one time.
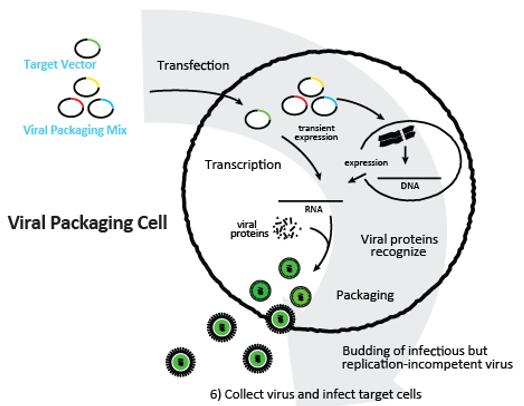
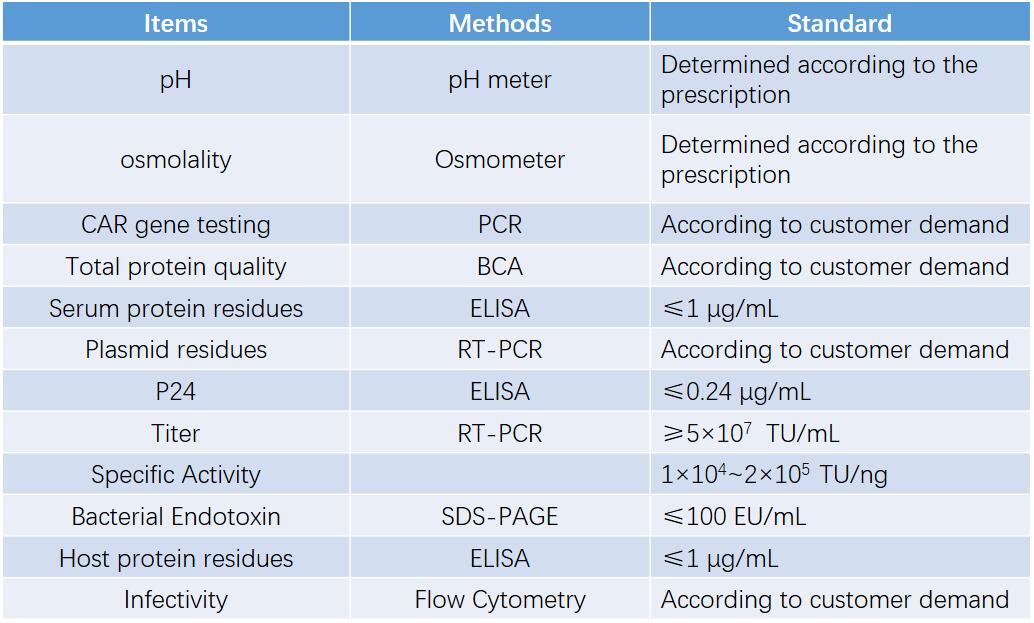
We also perform versatile measurements to make sure the quality of virus, including but not limited to items in the table below.
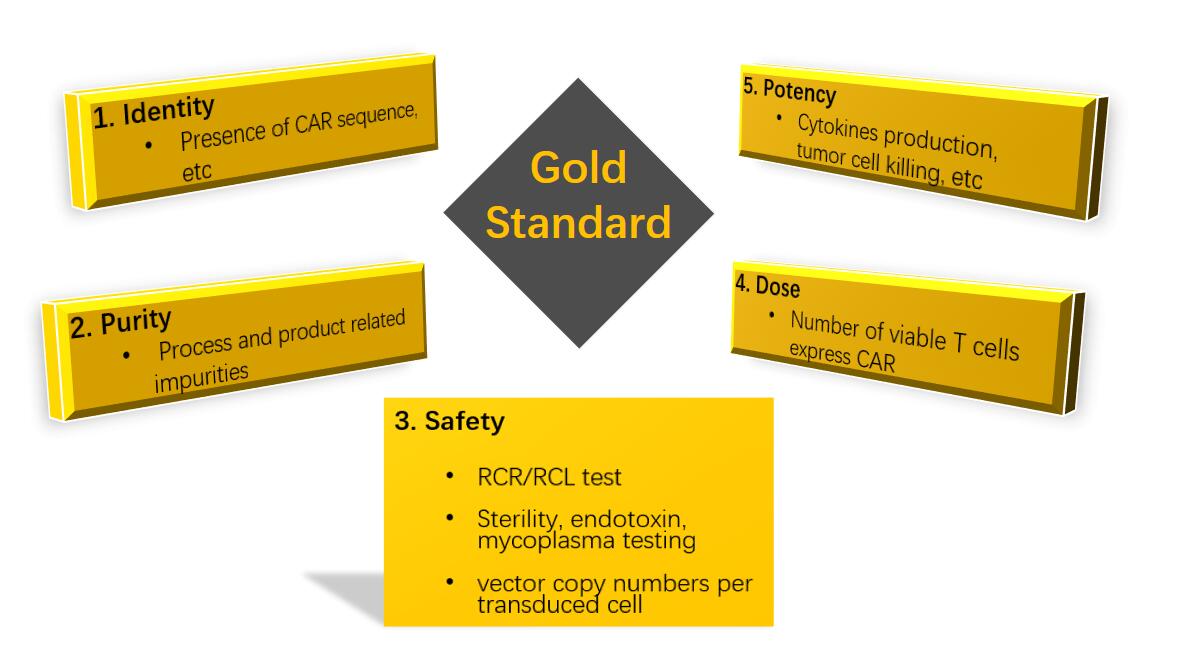
GMP-level CAR-T cell Production
CAR-T cell is the core of cancerous cell therapy. A stable cell line is of great importance for reproducibility of the experiment. The large-scale production of CAR-T cell can lead the revolution of overall application of cell therapy. Creative Biolabs has self-developed the technology of large-scale production of CAR-T cell. We have the ability to manufacture CAR-T cell under the premise of ensuring purity, safety, identity, dose, and potency.
Creative Biolabs has the capability to enable you to free up your time for core work and project. As a brilliant supplier of cell therapy products and technologies, Creative Biolabs can meet your special needs if you have any requirements. If you are interested in our service, please feel free to contact us and our team will get back to you as soon as possible.
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION