Chimeric antigen receptor (CAR) T cell therapy has revolutionized cancer immunotherapy with unprecedented success in hematological malignancies, yet significant challenges persist in treating solid tumors and expanding its therapeutic reach. CAR-NKT (Natural Killer T) cell therapy emerges as a next-generation approach, leveraging the unique properties of NKT cells to overcome traditional CAR-T limitations, particularly in addressing the complex challenges of solid tumor treatment. Our One-Stop CAR-NKT Therapy Development Solution provides comprehensive, integrated support across the entire development pipeline - from vector design and construction through preclinical validation to clinical trial advancement. Backed by state-of-the-art technologies and profound expertise in cell therapy development, we accelerate CAR-NKT programs and transform breakthrough concepts into clinical realities.
CAR-NKT cells represent a pioneering advancement in adoptive cell therapy, uniquely featuring a dual-targeting mechanism through both CAR and invariant T cell receptor (iTCR) pathways. This distinctive iTCR-CD1d interaction enables CAR-NKT cells to exhibit both CAR-dependent and CAR-independent cytotoxicity, significantly enhancing their anti-tumor potential. Beyond direct tumor targeting, CAR-NKT cells possess the remarkable ability to modulate the tumor microenvironment (TME) by targeting tumor-associated macrophages (TAMs) and myeloid-derived suppressor cells (MDSCs) through CD1d-dependent mechanisms. Importantly, the MHC-independent nature of iTCR activity contributes to a reduced risk of graft-versus-host disease (GVHD), offering a superior safety profile.
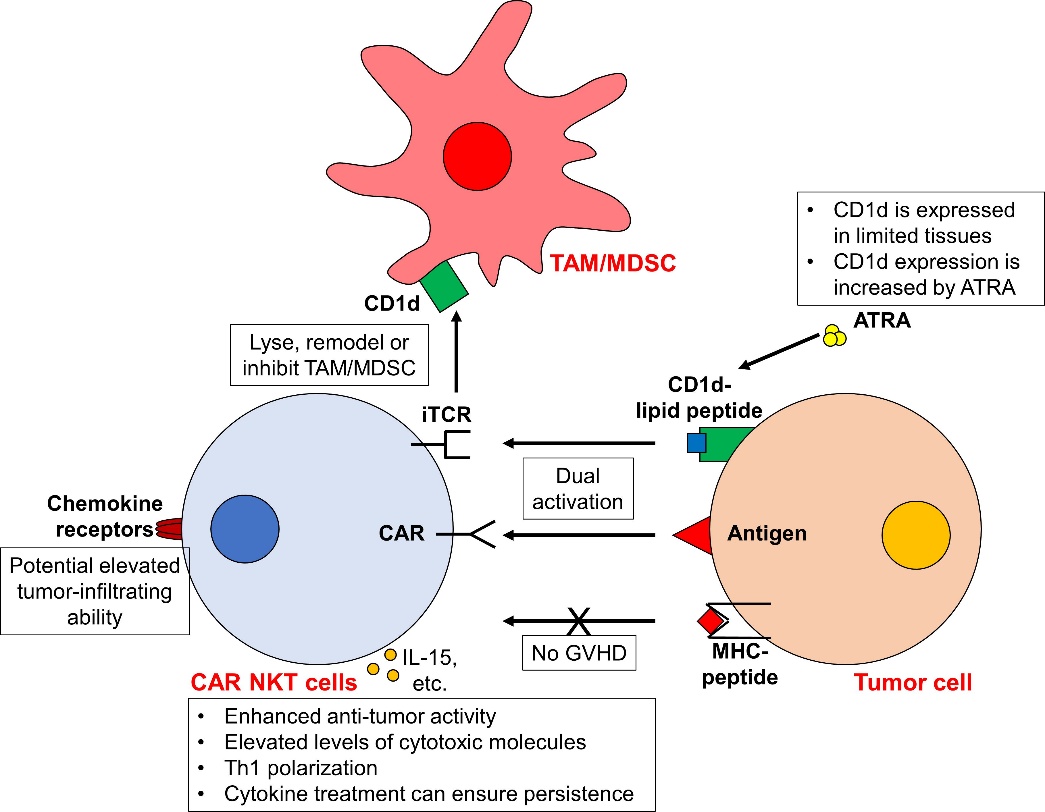 Fig.1 CAR NKT cells exhibit enhanced antitumor activity against tumor cells.1.
Fig.1 CAR NKT cells exhibit enhanced antitumor activity against tumor cells.1.
Compared to both CAR-T and CAR-NK approaches, CAR-NKT cells offer several distinct advantages. They demonstrate superior tumor infiltration through their innate tissue-penetrating abilities and chemokine-driven migration patterns. CAR-NKT cells maintain consistent cytokine profiles and exhibit lower risk of cytokine release syndrome (CRS), potentially offering a better safety profile than CAR-T cells. Additionally, their natural tumor-homing properties and ability to modulate the immunosuppressive TME make them particularly promising for solid tumor treatment. The cells' uniform phenotype and relatively straightforward manufacturing process also provide practical advantages over CAR-NK cells in terms of standardization and scalability.
Our vector design and construction service leverages cutting-edge computational tools and proprietary algorithms to create optimized CAR constructs specifically for NKT cells. We provide comprehensive solutions encompassing target epitope analysis, scFv humanization and affinity optimization, customized spacer/hinge design, and strategic selection of co-stimulatory domains. Our validated backbone vectors incorporate advanced safety elements including suicide genes, regulated expression systems, and optimized regulatory elements. Through systematic optimization of promoter strength, codon usage, and regulatory sequences, we ensure robust CAR expression while maintaining NKT cell fitness. Each vector undergoes rigorous quality control including sequence verification, expression analysis, and stability testing to guarantee optimal performance.
Our state-of-the-art viral vector production and gene delivery platform is specifically optimized for NKT cells. We offer viral packaging services delivering high-titer lentiviral or retroviral preparations with exceptional purity profiles (>90%) and validated potency. Our comprehensive testing includes sterility, endotoxin, mycoplasma, and RCL testing. Using our proprietary transduction protocols, we consistently achieve superior gene delivery efficiency (typically >70%) while preserving key NKT cell characteristics including CD1d restriction and innate anti-tumor functions. Our process incorporates optimized transduction conditions, enhanced viral entry mechanisms, and specialized culture systems to maintain cell viability and functionality throughout the genetic modification process.
Our extensive in vitro testing platform provides multi-parameter functional evaluation of CAR-NKT products. We conduct comprehensive analyses including cytotoxicity assays against multiple tumor lines, detailed cytokine profiling proliferation kinetics, and persistence studies using advanced flow cytometry and real-time monitoring systems. Our validated assays assess both CAR-mediated and innate NKT cell functions, including CD1d recognition, lipid antigen reactivity, and serial killing capacity. We employ sophisticated co-culture systems to evaluate CAR-NKT performance in the presence of immunosuppressive factors and assess potential exhaustion markers. Advanced imaging platforms enable real-time visualization of CAR-NKT-target cell interactions and killing dynamics.
We provide comprehensive preclinical evaluation using advanced humanized mouse models and cutting-edge imaging technologies. Our in vivo studies thoroughly assess CAR-NKT trafficking, tumor infiltration patterns, persistence, and anti-tumor efficacy using both subcutaneous and orthotopic tumor models. Sophisticated imaging techniques, including bioluminescence and multi-color flow cytometry, enable detailed tracking of CAR-NKT biodistribution and tumor response. We conduct extensive safety assessments including cytokine release evaluation, off-tumor toxicity studies, and long-term safety monitoring. Our comprehensive analysis includes tumor microenvironment modulation, immune cell interactions, and development of resistance mechanisms, generating robust data packages for IND applications.
Our clinical trial support service provides end-to-end expertise for transitioning CAR-NKT therapies into clinical studies. We offer comprehensive support in clinical protocol development, regulatory documentation preparation, and manufacturing process establishment. Our team assists with all aspects of IND filing, including detailed CMC documentation, process validation, stability studies, and clinical trial design. We provide expertise in patient eligibility criteria definition, clinical endpoint selection, and safety monitoring strategies. Our support extends to developing SOPs, quality control metrics, and release criteria for clinical-grade CAR-NKT products. We ensure compliance with current regulatory requirements while optimizing the path to clinical implementation through risk-based approaches and strategic regulatory planning.
Our state-of-the-art CAR-Stem Cell Platform represents a breakthrough in iPSC-NKT cell manufacturing technology, combining the power of induced pluripotent stem cells with the therapeutic potential of CAR-NKT cells. The platform employs proprietary reprogramming protocols to generate NKT cell-derived iPSCs (NKT-iPSCs) from carefully selected healthy donors, establishing a renewable source of therapeutic cells with unlimited expansion capacity. Through precise genetic engineering and optimized culture conditions, we maintain the essential characteristics of parent NKT cells throughout the reprogramming process.
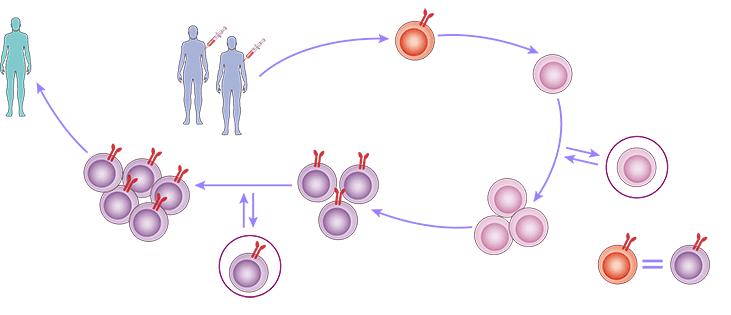 Fig 2. Workflow for our iPSC-derived NKT cell platform.
Fig 2. Workflow for our iPSC-derived NKT cell platform.
Our platform's validated differentiation protocol leverages specific cytokine combinations and optimal culture conditions to efficiently direct NKT-iPSCs toward functional iPSC-NKT cells. These regenerated cells undergo rigorous quality control to ensure the preservation of key NKT cell features, including CD1d restriction and cytokine profiles, while exhibiting enhanced CAR-directed tumor recognition and killing capacity. This innovative approach enables the production of standardized, off-the-shelf CAR-NKT cell products suitable for treating multiple patients, offering a scalable solution for cell therapy development.
We specialize in designing optimized CAR constructs specifically for NKT cells. Our service includes customization of key components including: 1) Second and third-generation CARs with validated co-stimulatory domains (CD28, 4-1BB) optimized for NKT cell biology, 2) Tailored spacer lengths based on target epitope location, and 3) Proprietary promoter elements ensuring stable CAR expression while maintaining NKT functionality. We also offer comprehensive vector optimization including codon optimization and regulatory element selection based on your specific requirements.
Our functional evaluation package includes: 1) Multi-parameter cytotoxicity assays against both CD1d+ and CD1d- tumor lines with real-time killing kinetics, 2) Comprehensive cytokine profiling (IFN-γ, IL-4, IL-13, GM-CSF) using multiplexed assays, 3) Long-term persistence studies with weekly phenotype and function assessment up to 28 days, 4) Exhaustion marker analysis (PD-1, LAG-3, TIM-3), and 5) Memory phenotype characterization. All assays are validated with reference standards and include detailed statistical analysis.
Our comprehensive QC package includes: 1) Viral titer determination using qPCR and flow cytometry, 2) Sterility testing including mycoplasma, endotoxin, and bacterial/fungal contamination, 3) Sequence verification of the packaged transgene, 4) Functional testing of viral particles using reporter cell lines, and 5) Stability assessment at different storage conditions. We provide detailed COA documentation meeting regulatory requirements for both research and clinical applications.
We implement multiple control measures throughout the manufacturing process: 1) Standardized cell isolation and activation protocols optimized for NKT cells, 2) Real-time monitoring of cell phenotype (including CD1d restriction and CAR expression) during expansion, 3) Regular testing of cytokine production profiles and cytotoxicity against reference targets, and 4) Validated cryopreservation protocols maintaining >80% post-thaw viability and functionality.
Our platform incorporates multiple approaches to optimize CAR-NKT performance in solid tumors: 1) Proprietary CAR designs incorporating chemokine receptors matched to tumor-specific chemokines, 2) Optimization of CD1d-dependent tumor recognition through ATRA-mediated CD1d upregulation, 3) Enhanced expansion protocols utilizing specific cytokine combinations (IL-15/IL-21) to promote memory phenotype development, and 4) Validated administration strategies including optimal dosing schedules and IL-15 support for sustained persistence.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION