Chimeric Antigen Receptor (CAR) T-cell therapy is a groundbreaking immunotherapy that has demonstrated remarkable success in the treatment of hematological malignancies. Traditionally, CAR-T therapies utilized viral vectors for gene delivery, raising concerns about safety and manufacturing challenges. In contrast, mRNA CAR-T therapy employs a non-viral approach, capitalizing on the transient expression of CAR molecules in T-cells.
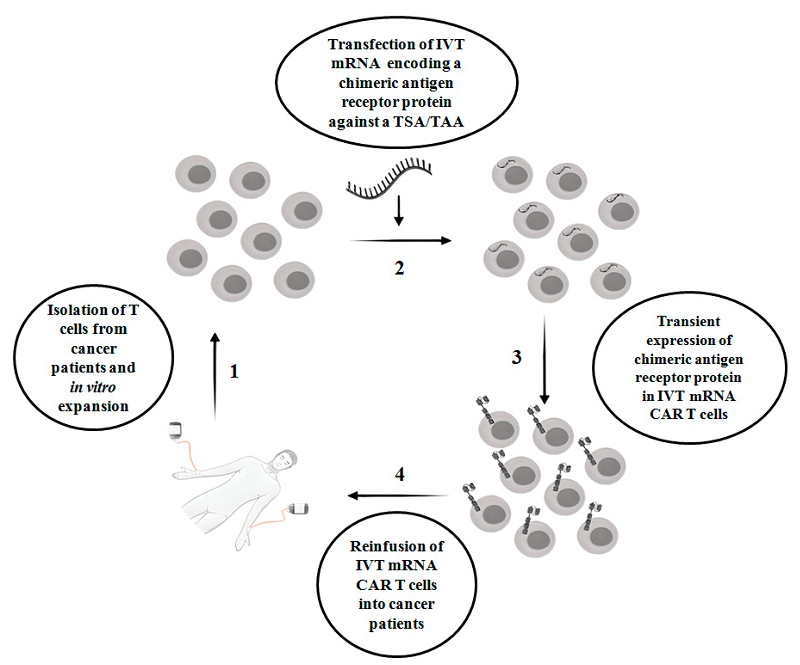 Figure 1. The scheme of IVT mRNA CAR T therapy in cancer patients.1
Figure 1. The scheme of IVT mRNA CAR T therapy in cancer patients.1
Our mRNA CAR-T therapy leverages the simplicity and safety of mRNA, offering several pivotal advantages that distinguish it from traditional approaches.
To comprehend mRNA CAR-T therapy, it is essential to understand the core component – in vitro transcribed (IVT) mRNA. IVT mRNA is a synthetic version of natural mRNA, engineered to express the desired CAR constructs. Creative Biolabs specializes in the design and production of high-quality IVT mRNA vectors tailored to your specific target antigen.
Our team of experts is equipped with the expertise to design and optimize the IVT mRNA vector, ensuring maximal CAR expression, minimized off-target effects, and enhanced translational efficiency. These vectors can be readily modified to target different antigens, demonstrating the versatility of mRNA CAR-T technology.
One of the key challenges in mRNA-based therapy is the efficient delivery of mRNA to target cells. Creative Biolabs addresses this challenge with the utilization of Lipid Nanoparticles (LNPs). LNPs are lipid-based carriers that protect the fragile mRNA cargo, enhance cellular uptake, and facilitate endosomal escape, thus ensuring optimal translation of the CAR protein.
Our proprietary LNP formulations have been meticulously developed to achieve high delivery efficiency while minimizing cytotoxicity. This expertise empowers us to offer a streamlined and highly effective mRNA CAR-T therapy development service.
Creative Biolabs' One-Stop mRNA CAR-T Therapy Development service encompasses a streamlined, efficient, and end-to-end workflow that facilitates the development of customized mRNA CAR-T therapies.
The advantages of mRNA CAR-T therapy over traditional viral vector-based CAR-T therapies are significant. These advantages include:
In conclusion, mRNA CAR-T therapy represents a revolutionary leap forward in the field of immunotherapy. Its safety, flexibility, and rapid development potential make it a promising avenue for cancer treatment. Creative Biolabs is committed to advancing the field through our expertise and the One-Stop mRNA CAR-T Therapy Development service. Together, we are shaping the future of cancer therapy.
For more information on our service and to explore how we can collaborate on your next project, please get in touch with our expert team.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION