Chimeric antigen receptors (CARs) are designed to attach to specific proteins to reprogram T cells to kill cancer. The success of CAR-T cell therapy highlights the prospect of programmed immunity and suggests that applying CAR strategies to other immune cell lineages may be beneficial. As a leading technology provider, Creative Biolabs has established the CellRapeutics™ chimeric engulfment receptor (CER) technology platform for phagocytosis, which directs macrophages to engulf specific targets on cancer cells.
Significance of Macrophage-mediated Phagocytosis
Macrophages are a key effector of the innate immune system and are responsible for engulfing debris and pathogens. A variety of evidence shows that macrophages have the unique ability to penetrate solid tumors and fight tumor growth, while other immune cells (such as T cells) are physically rejected or inactivated. This suggests that engineered macrophages may enhance existing T cell-based cell therapies.
Another supporting finding is that antibody blocking of the negative regulator of phagocytosis, CD47, reduces tumor burden, suggesting that metastatic balance to promote macrophage activation and phagocytosis is a promising therapeutic approach. Therefore, our innovative CER-macrophage therapy holds broad therapeutic prospects in the future.
CER Design and Construction
We have developed different generations of CERs. Similar to CAR, our CERs essentially consist of three modules: the extracellular domain targeting (ECD) targeting the phagocytic target antigen, transmembrane domain (TMD) and phagocytosis intracellular signaling domain (ESD). The ECD protein can be scFv, Fab, peptide, or sometimes an FcR. The ESD of our second-generation CER includes a primary phagocytic signal domain and a secondary phagocytic signal domain.
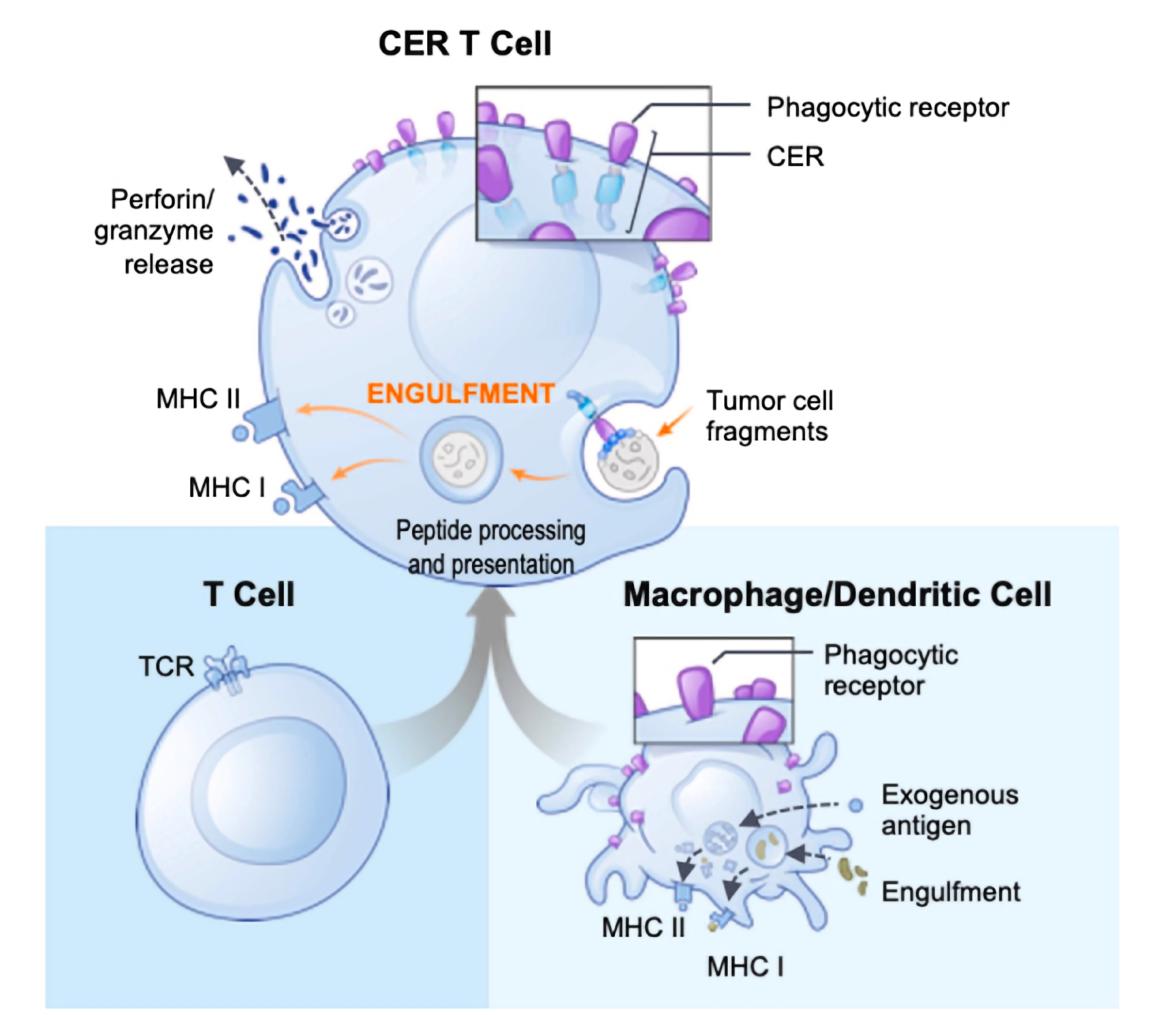 Fig.1 Schematic of the structure of CER-T cell.1
Fig.1 Schematic of the structure of CER-T cell.1
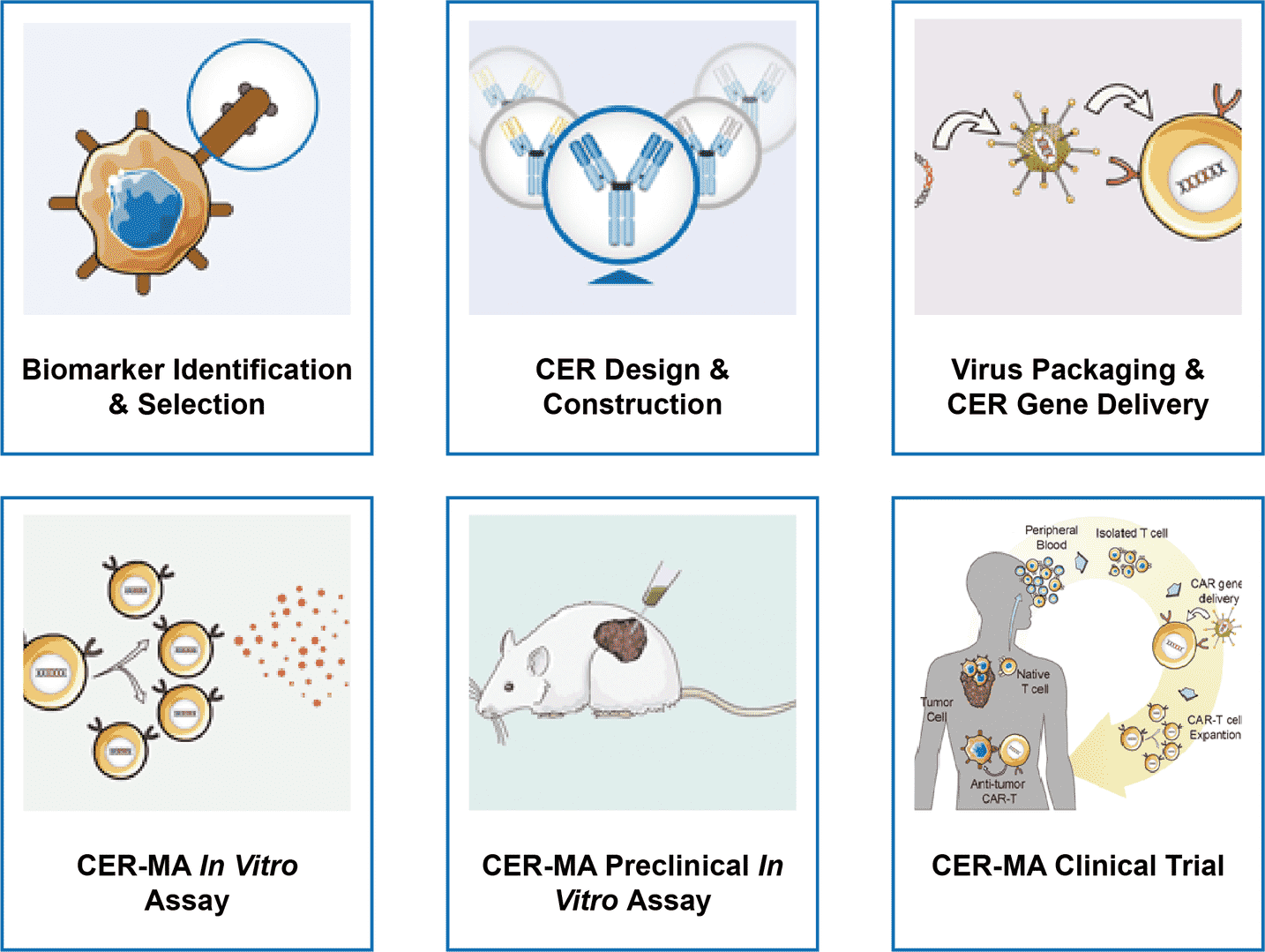
One-stop CER-MA Development Services
Empowered by our high qualified groups and advanced technologies, Creative Biolabs provides one-stop CER-MA development services:
It should be noted that our CER can not only genetically modify macrophages, but also modify the phagocytic phenotype of cells that do not naturally show phagocytic activity, including T cells, natural killer cells, natural killer T cells, B cells, somatic cells, dendritic cells, Langerhans cells, and bone marrow cells.
For more detailed information, please feel free to contact us or directly sent us an inquiry.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION