Creative Biolabs' PCL platform takes viral vector manufacturing to the next level!
Viral vectors are useful experimental tools for stable gene delivery and have been used to treat cancer in immunotherapy (such as chimeric antigen receptor T cell therapy, CAR-T). Lentiviral vectors are usually produced using a transient process of transfecting plasmid DNA into production cells. However, this method is expensive, has poor reproducibility, and is difficult to scale up. Manufacturing large amounts of materials for later clinical trials and product commercialization is still a challenge using this process.
The production of stable cell lines facilitates a GMP-compliant process, providing easier scale-up, reproducibility, and cost-effectiveness. At Creative Biolabs, we have developed a highly efficient Stable Packaging Cell Line (PCL) Platform specifically designed for the CAR virus vector scalable production. Through a proprietary packaging and producer cell lines optimization strategy, our PCL platform enables high-performance virus vector production from preclinical research-grade and smaller amounts up to large scale production in significantly reduced timelines. In addition, our PCL platform enables consistent quality virus delivery, cost-effective manufacturing, and minimize process risks. No need for helper viruses, expensive transfection reagents, or cGMP-grade plasmids. Just propagate our packaging cell lines and switch on production.
Viral vectors have been widely used for gene transfer, and each virus has its unique functions, making it suitable for specific treatments. We have used inducible and constitutive systems to generate multi-suspension and adhesion-based stable lentiviral vector packaging cell lines. In addition to lentiviral vectors, we also provide effective host cell lines for the production of AAV and adenoviral vectors. These cell lines can be used not only for the large-scale production of CAR viruses, but also for the production of viral vaccines, oncolytic viruses, and exosomes.
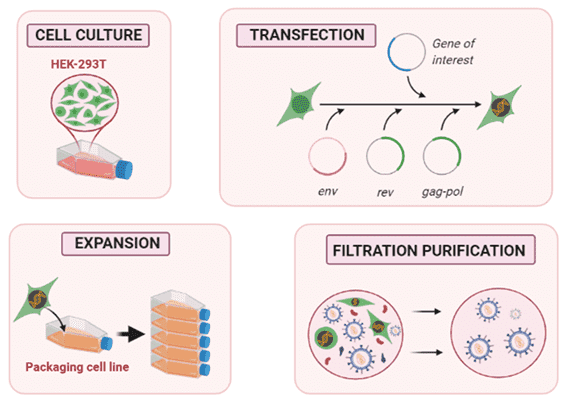 Fig.1 Proposed steps for lentiviral vector production in a packaging cell line.1
Fig.1 Proposed steps for lentiviral vector production in a packaging cell line.1
If you are interested in our services, please send an email to contact us, and our team will get back to you as soon as possible.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION