NK cells function similarly to cytotoxic T cells in the innate immune response. With the development of CAR-T cells, CAR-NK cells have been widely studied along with their superior advantages, for example, NK cells with MHC class I independent recognition enable them to maintain an attack on tumor cells with reduced MHC expression. In the context of the potential of in vivo CAR therapy to advance the field of cell therapy, establishing an in vivo CAR-NK cell engineering service to generate CAR-NK cells in situ will be of great significance for the treatment of cancer and other diseases.
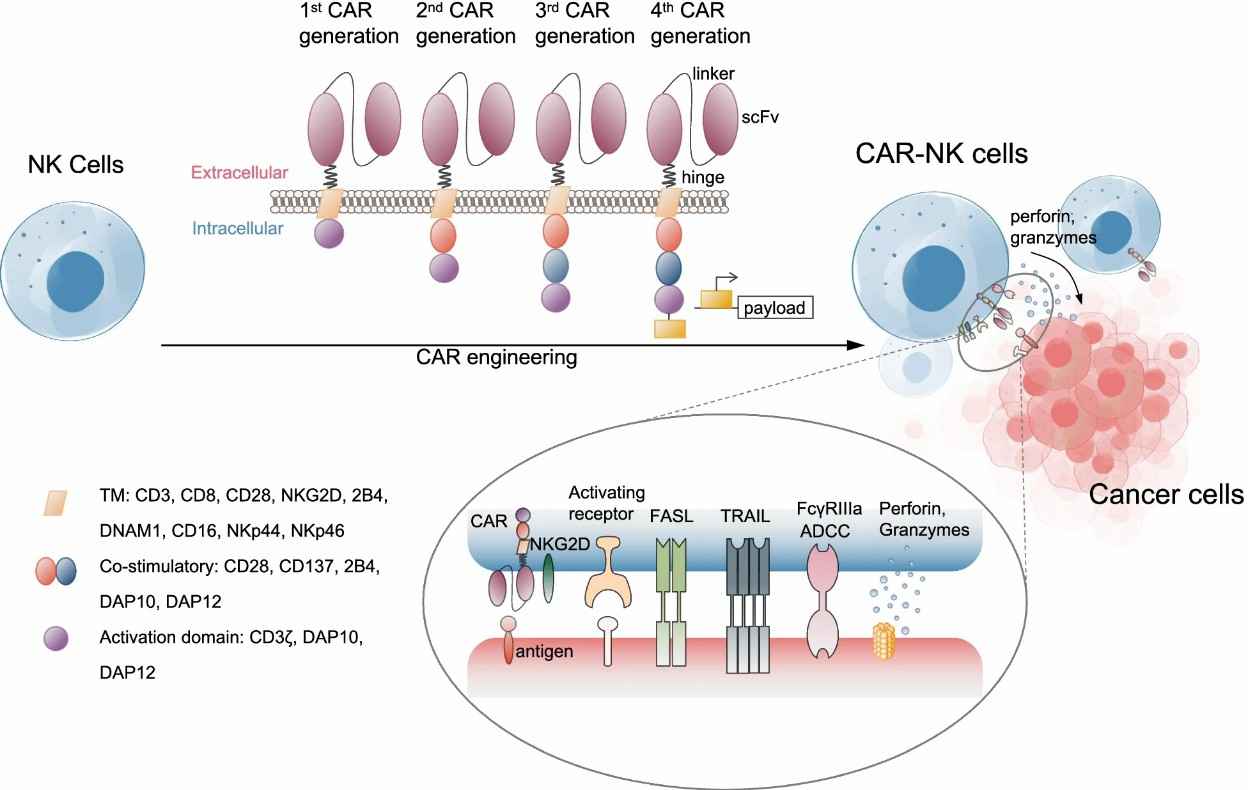 Fig.1 The overview of the constructs and targets of CAR-NK cells. (Zhang, et al., 2022)
Fig.1 The overview of the constructs and targets of CAR-NK cells. (Zhang, et al., 2022)
After years of research on in vivo cell engineering, Creative Biolabs has established a safe and cost-effective in vivo CAR-NK cell service platform. Since the transfection efficiency of NK cells using viral vectors is generally lower than that of T cells under normal conditions, we used a variety of materially optimized vectors as transfection tool alternatives, such as PEI-coated magnetic NPs used for in vivo CAR-NK Cell Engineering. At the same time, we are committed to offering a customized service that encompasses everything from CAR design to function verification to meet the varying needs of our global customers.
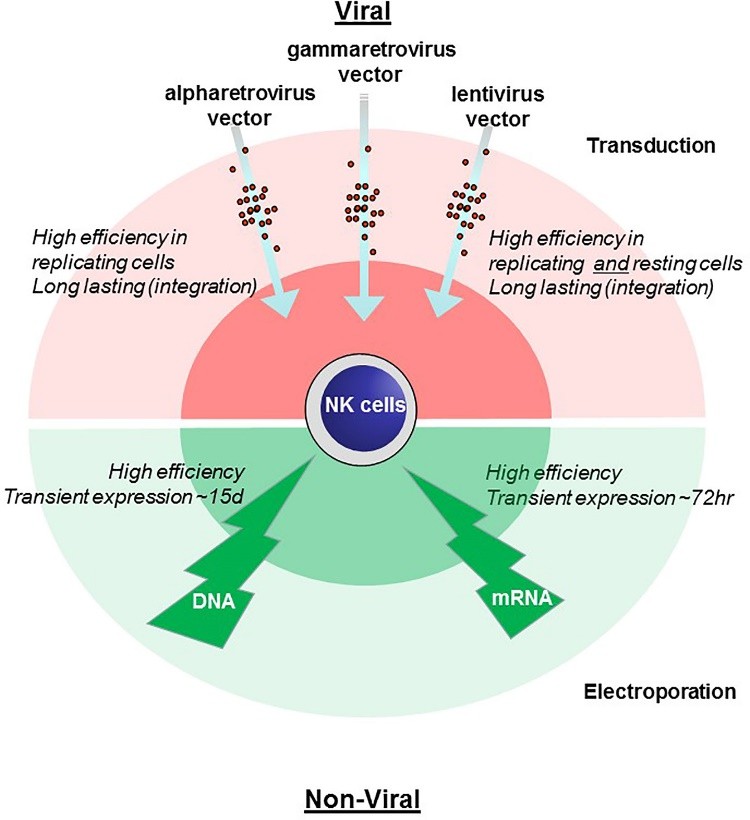 Fig.2 Methods Comparison for genetic engineering of NK cells. (Schmidt, et al., 2021)
Fig.2 Methods Comparison for genetic engineering of NK cells. (Schmidt, et al., 2021)
Representative data: CAR-NK cell-mediated immunotherapy has expanded rapidly and has emerged as a viable therapeutic option for patients with advanced cancer.
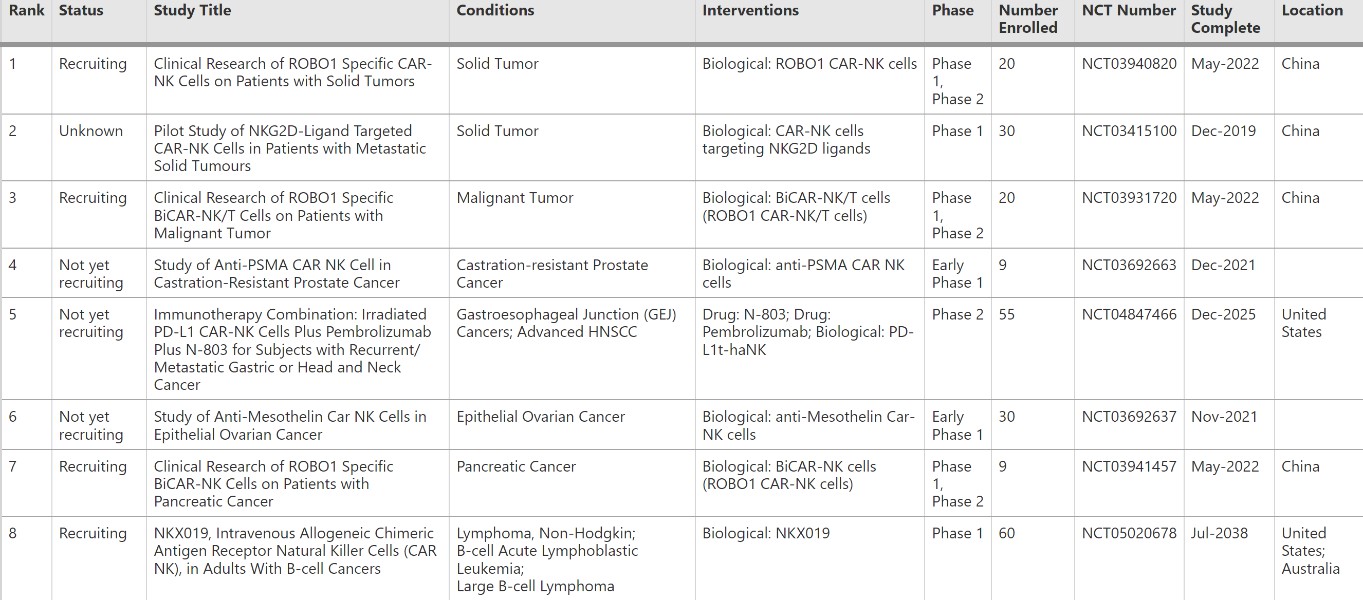 Fig.3 Several clinical trials of CAR-NK cell-based cancer immunotherapy. (Zhang, et al., 2022)
Fig.3 Several clinical trials of CAR-NK cell-based cancer immunotherapy. (Zhang, et al., 2022)
We provide inquiry modes as follows that allow more detailed discussions with us.
In the field of in vivo CAR-NK cell engineering, we are devoted to discovering novel approaches to dramatically improve its efficacy in cancer therapy. At the same time, we also provide the following related services:
Frequently Asked Questions
Q: What is the difference between the CAR-NK cell and CAR-T cell therapy?
A: Although both CAR NK cell and CAR-T cell treatments utilize customized immune cells to target and destroy cells expressing a particular antigen, there are significant variations between the two therapies. CAR-T cells, the first FDA-approved CAR-engineered cellular immunotherapy, have a lengthy clinical history. Allogeneic T cells may cause graft-versus-host disease, whereas allogeneic NK cells do not, allowing for NK cell therapy. Allogeneic CAR NK cells are less likely than autologous CAR-T cells to produce cytokine release syndrome (CRS), a potentially lethal condition caused by IL-6, IL-1, etc.
For more details about our in vivo CAR-NK cell engineering service, please don't hesitate to contact us. We are sincerely looking forward to working with global customers.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION