Chimeric antigen receptor-modified regulatory T cells (CAR-Tregs) represent a groundbreaking frontier in cellular immunotherapy, combining the specificity of CAR technology with the powerful immunosuppressive capabilities of regulatory T cells. This innovative therapeutic approach holds immense promise for treating autoimmune diseases, preventing transplant rejection, and managing various inflammatory conditions. By engineering Tregs to express CARs specific to desired antigens, these cells can provide targeted immunosuppression while maintaining immune homeostasis in specific tissues, offering superior therapeutic potential compared to conventional treatments.
At Creative Biolabs, we leverage our extensive expertise in CAR technology and immunology to offer comprehensive CAR-Treg development services. Our end-to-end solutions, backed by state-of-the-art facilities and experienced scientists, enable partners to create CAR-Tregs products that support the next generation of cell therapy innovation.
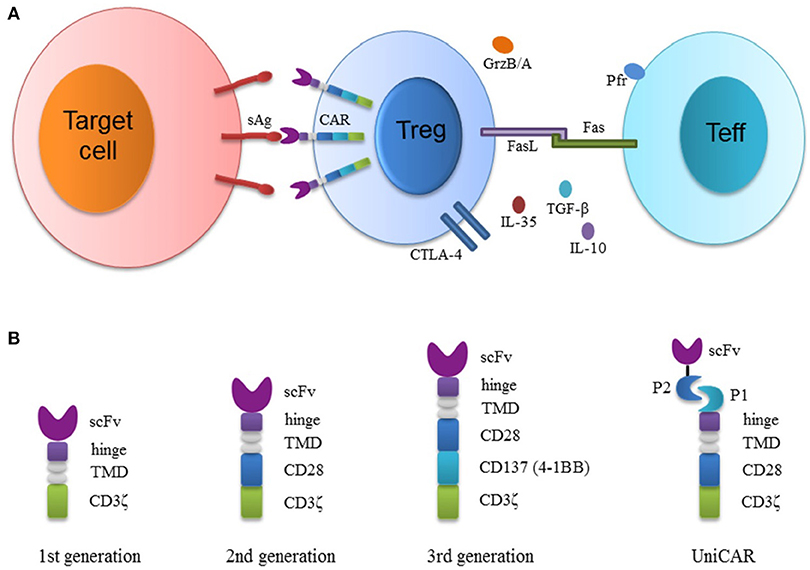 Fig. 1 Schematic diagram depicting the structure of CAR-Tregs and their suppression of effector T (Teff) cells.1
Fig. 1 Schematic diagram depicting the structure of CAR-Tregs and their suppression of effector T (Teff) cells.1
The distinct functional variations between conventional CAR-T cells and CAR-Treg cells present unique opportunities for treating different diseases. Below, we examine the fundamental differences between these therapeutic approaches, highlighting their distinct characteristics, challenges, and advantages.
Derived from effector T cells (CD8+ cytotoxic T cells), engineered to recognize and eliminate specific target cells through direct cytotoxicity.
Direct cytotoxicity through perforin and granzyme release, accompanied by pro-inflammatory cytokine production (IFN-γ, TNF-α), leading to potential cytokine release syndrome (CRS).
Primarily successful in treating hematological malignancies such as refractory acute lymphoblastic leukemia and relapsed/refractory non-Hodgkin's lymphoma, with ongoing clinical trials exploring applications in solid tumors.
Engineered from regulatory T cells (CD4+ CD25+ FOXP3+), specifically designed for maintaining immune tolerance and homeostasis.
Targeted immunosuppression through anti-inflammatory cytokines (IL-10, TGF-β), with direct suppression of effector T cells and modulation of antigen-presenting cells.
Specifically designed for autoimmune diseases, transplant rejection prevention, and inflammatory conditions, offering targeted immunosuppression.
From initial biomarker identification to clinical trial support, we offer a highly customizable service workflow tailored to your specific requirements. Our experienced team works closely with you at each step to ensure optimal results for your CAR-Treg development program. Below are the key services we provide, which can be customized based on your unique needs.
Comprehensive target antigen identification and validation, followed by development of specific scFv.
Expert design and optimization of CAR constructs tailored for Treg cell engineering.
Efficient viral and non-viral delivery systems for optimal CAR gene transfer.
Comprehensive in vitro testing to validate CAR-Treg functionality and efficacy.
Rigorous preclinical in vivo studies to evaluate safety and efficacy in animal models.
Comprehensive support for clinical trial development and implementation.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION