Are you currently facing challenges such as the high cost and logistical complexities of autologous cell therapies, or the limited scalability and persistence of primary NK cell products? Our iPSC-derived CAR-NK therapy development solution helps you accelerate the creation of highly effective, standardized, and scalable allogeneic cellular immunotherapies through advanced iPSC engineering and comprehensive development platforms.
The great success of chimeric antigen receptor (CAR)-T cells has prompted scientists to genetically modify human natural killer (NK) cells with CAR to better treat lethal cancer. NK cells can now be routinely produced from human pluripotent stem cells (PSC). These PSCs provide a novel resource that can be used to produce "off-the-shelf" NK cells with a homogeneous and standardized group for CAR-NK therapy.
Induced PSC (iPSC)-derived CAR-NK therapy represents a transformative leap in cellular immunotherapy. By leveraging the unique self-renewal and differentiation capabilities of iPSCs, this solution provides an unlimited, standardized, and genetically engineerable source of NK cells. This addresses critical limitations of traditional CAR-T and primary NK cell therapies, such as manufacturing complexity, donor variability, and limited persistence.
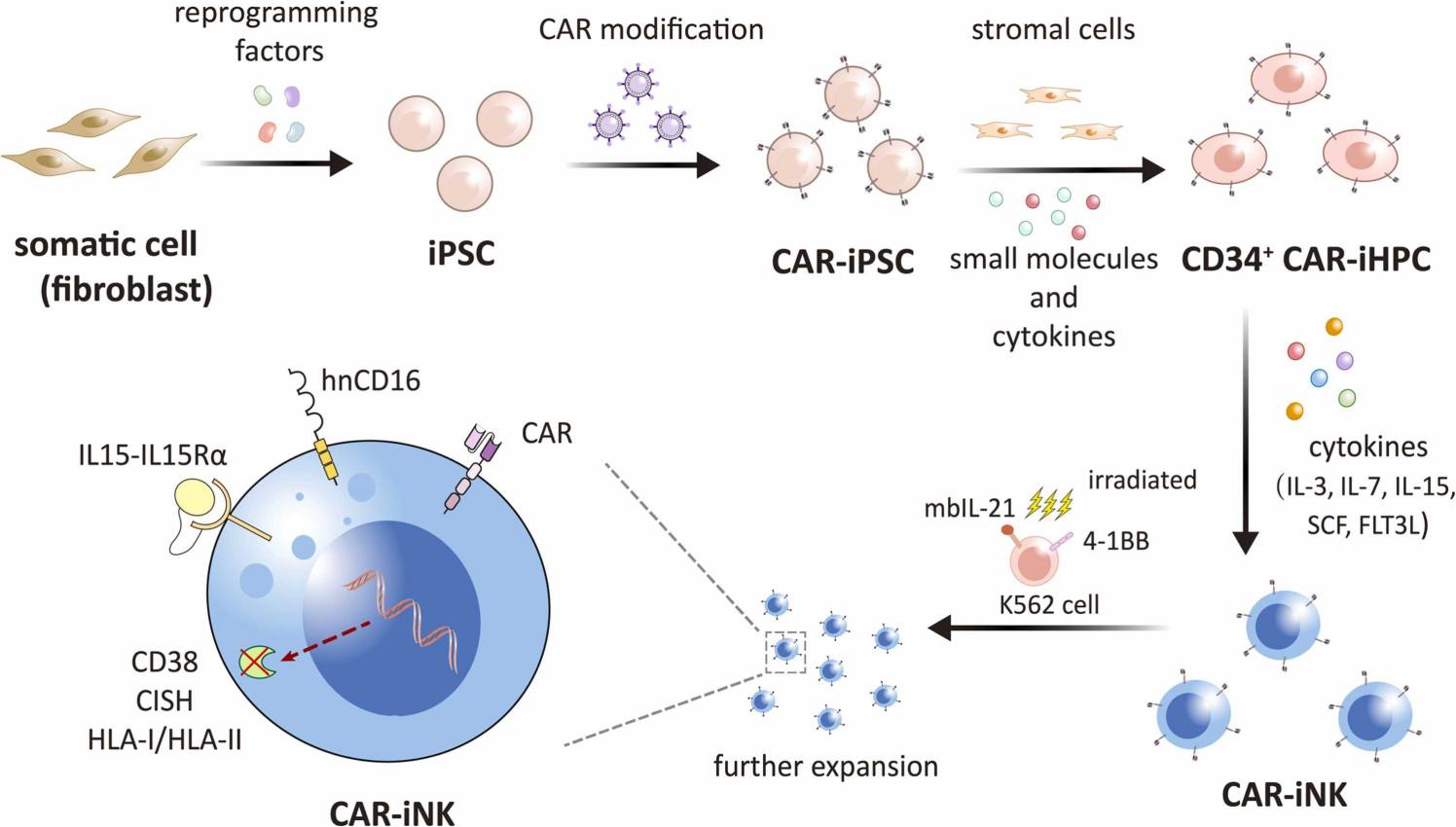 Fig.1 Produce iPSC-derived natural killer cells expressing CAR.1
Fig.1 Produce iPSC-derived natural killer cells expressing CAR.1
At Creative Biolabs, we have developed the iPSC technology platform to produce NK cells derived from CAR-expressing stem cells. This technology platform provides an unlimited source of engineered stem cell-derived CAR-NK immune cells. These PSC-derived NK cells have similar phenotypes and functions to primary lymphocytes isolated from peripheral blood, thereby they can be precisely engineered to introduce the improved anti-tumor activity.
We provide multiple strategies to engineer iPSC-derived NK (iPSC-NK) cells for enhanced functional potential, persistence, and homing. Examples of such gene editing strategies include: (i) strategies aimed at introducing specificity through cleavage-resistant CD16 to combine with therapeutic antibodies or CAR; (ii) strategies aimed at improving the persistence of allogeneic iPSC-NK cells by making cells self-sufficient in terms of growth factors; (iii) strategies aimed at co-expressing CXCR3 to enhance the iPSC-NK cell homing to tumor cells. More customized gene editing strategies can be performed according to your requirements.
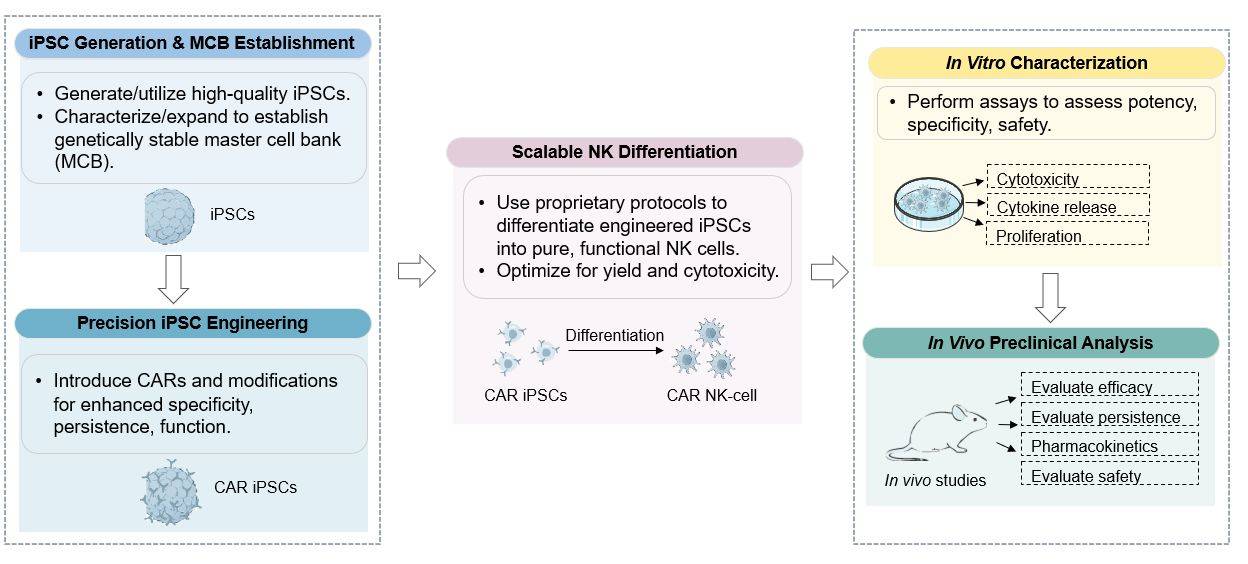
As the leading cell therapeutics biotech that provides cell therapy-related services, Creative Biolabs provides one-stop services for CAR-NK therapy development, including but not limited to:
Q1: What makes iPSC-derived CAR-NK cells superior to primary NK cells for therapeutic applications?
A1: iPSC-derived CAR-NK cells offer unparalleled advantages in scalability, homogeneity, and genetic engineerability. Unlike primary NK cells, which are limited by donor variability and finite expansion, iPSCs provide an unlimited, standardized source. This allows for consistent, large-batch manufacturing and precise genetic modifications to enhance anti-tumor activity, persistence, and minimize alloreactivity, leading to a more reliable and potent therapeutic product.
Q2: How does Creative Biolabs ensure the safety of its iPSC-derived CAR-NK products?
A2: Safety is paramount in our development process. We employ rigorous genetic engineering strategies, including knocking out genes associated with alloreactivity and incorporating safety switches. Our comprehensive in vitro and in vivo safety assessments, including tumorigenicity studies and monitoring for cytokine release syndrome (CRS), immune-effector-cell-associated neurotoxicity syndrome, and graft-versus-host disease, ensure a favorable safety profile.
Creative Biolabs stands at the forefront of iPSC-derived CAR-NK therapy development, offering unparalleled expertise and a proven track record. Our commitment to innovation, quality, and client success sets us apart, ensuring your project benefits from the most advanced technologies and scientific insights.
"The ability to generate homogeneous, large-scale batches of iPSC-derived CAR-NK cells through Creative Biolabs' platform has fundamentally transformed our manufacturing strategy, solving our long-standing issues with primary NK cell expansion. Their solution facilitated a seamless transition from lab-scale to industrial-scale production." - Prof. M***a S.
"Creative Biolabs' expertise in engineering dual-targeting CAR-NK cells has been instrumental in addressing the heterogeneous nature of our target disease. Their solution delivered a product with potent and sustained cytotoxicity against multiple antigens, providing a more comprehensive therapeutic approach than single-target strategies." - Dr. R***el K.
Creative Biolabs is dedicated to advancing the field of cellular immunotherapy through innovative iPSC-derived CAR-NK solutions. Our team of experts is ready to collaborate with you to overcome challenges and accelerate your therapeutic development.
Contact our team for more information and to discuss your project.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION