The remarkable efficacy of anti-CD19 chimeric antigen receptor (CAR) -T cell therapy in patients with leukemia and lymphoma has led to unprecedented response rates, demonstrating the clinical importance of genetically modified T cells as immunotherapy. Despite this clinical success, FDA-approved T-cell therapies are currently limited to B-cell malignancies and challenges remain in managing cytokine-related toxicity.
As a leading cell therapeutics provider, Creative Biolabs has established a novel CellRapeutics™ antibody-TCR (AbTCR) technology platform for the next generation of cell-based therapy. By combining the Fab domain of the antibody with the gamma and delta chains of TCR as effector domains, we demonstrated that AbTCR activates a cytotoxic T cell response similar to conventional CD3ζ-CAR, but with less cytokine release. Therefore, this revolutionary combination product holds huge potential and is expected to become an alternative therapy for cancer treatment.
Classical TCR versus CAR
T cells are defined by TCR molecules present on the surface of their cells. TCR promotes tumor immune surveillance by enabling T cells to recognize abnormal cells and triggering a series of signal events that lead to T cell activation and subsequent cancer cell lysis. In most T cells, TCR consists of alpha and beta chains, while in 1-5% of T cells, TCR consists of gamma (γ) and delta (δ) chains. Antigen-mediated activation of the αβ chains (or γδ chains) induces downstream signaling.
The chimeric antigen receptor borrows the hypervariable portion of a tumor-specific antibody and combines it into a single-chain hypervariable fragment (scFv) to confer CAR specificity. The design of CAR includes single-chain variable fragments with antigen-binding affinity fused to the spacer and transmembrane domains. Effector functions are conferred through the TCR CD3ζ domain, and the addition of a (2nd generation) or 2nd (3rd generation) co-stimulatory domain can drive signal activation and amplification of various effector signal cascades.
To date, effective single-chain designs have proven clinical efficacy as the foundation of most CAR-T therapies. However, the direct fusion of antigen recognition and cell activation domains produces a synthetic activation signal that may differ from the cell activation signal transmitted from the endogenous TCR-CD3 complex.
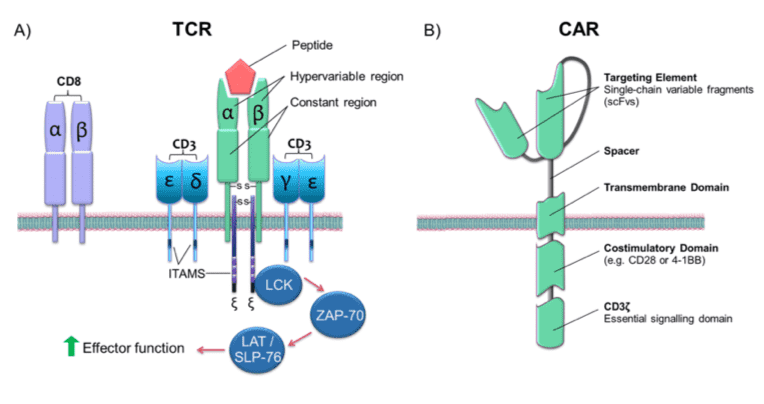 Fig.1 Structure of TCRs and CARs.1,3
Fig.1 Structure of TCRs and CARs.1,3
AbTCR Design and Construction
Based on our AbTCR platform, we designed and characterized a two-chain AbTCR. Considering there were technical hurdles with the mispairing with the T-cell 's endogenous α and β TCR chains, our AbTCR platform avoids mismatches by using the transmembrane and intracellular domains of γδTCR. More specifically, our synthetic AbTCR fuses the antigen-binding domain of an antibody with the signal domain of γδTCR. Similar to exogenously expressed TCR in TCR-T cells, our AbTCR engages endogenous CD3 complexes to initiate T cell activation. Since the γδTCR chain does not bind to TCR in αβT cells, AbTCR avoids the pairing errors of traditional αβTCR-based synthetic receptors. In addition, AbTCR's Fab antigen-binding domain can be used to target peptide-MHC complexes or cell surface antigens by using TCR-mimetic antibodies or conventional antibodies, respectively. Overall, the AbTCR design combines the antigen-binding utility of an antibody with an endogenous TCR activation pathway.
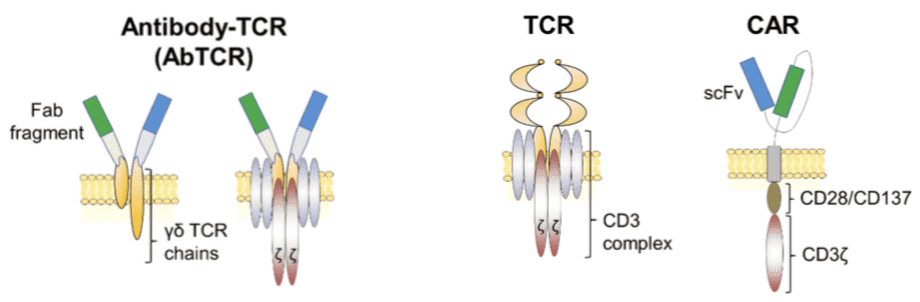 Fig.2 Schematic of antibody-TCR (AbTCR) platform compared to classical TCR and CAR platform.2,3
Fig.2 Schematic of antibody-TCR (AbTCR) platform compared to classical TCR and CAR platform.2,3
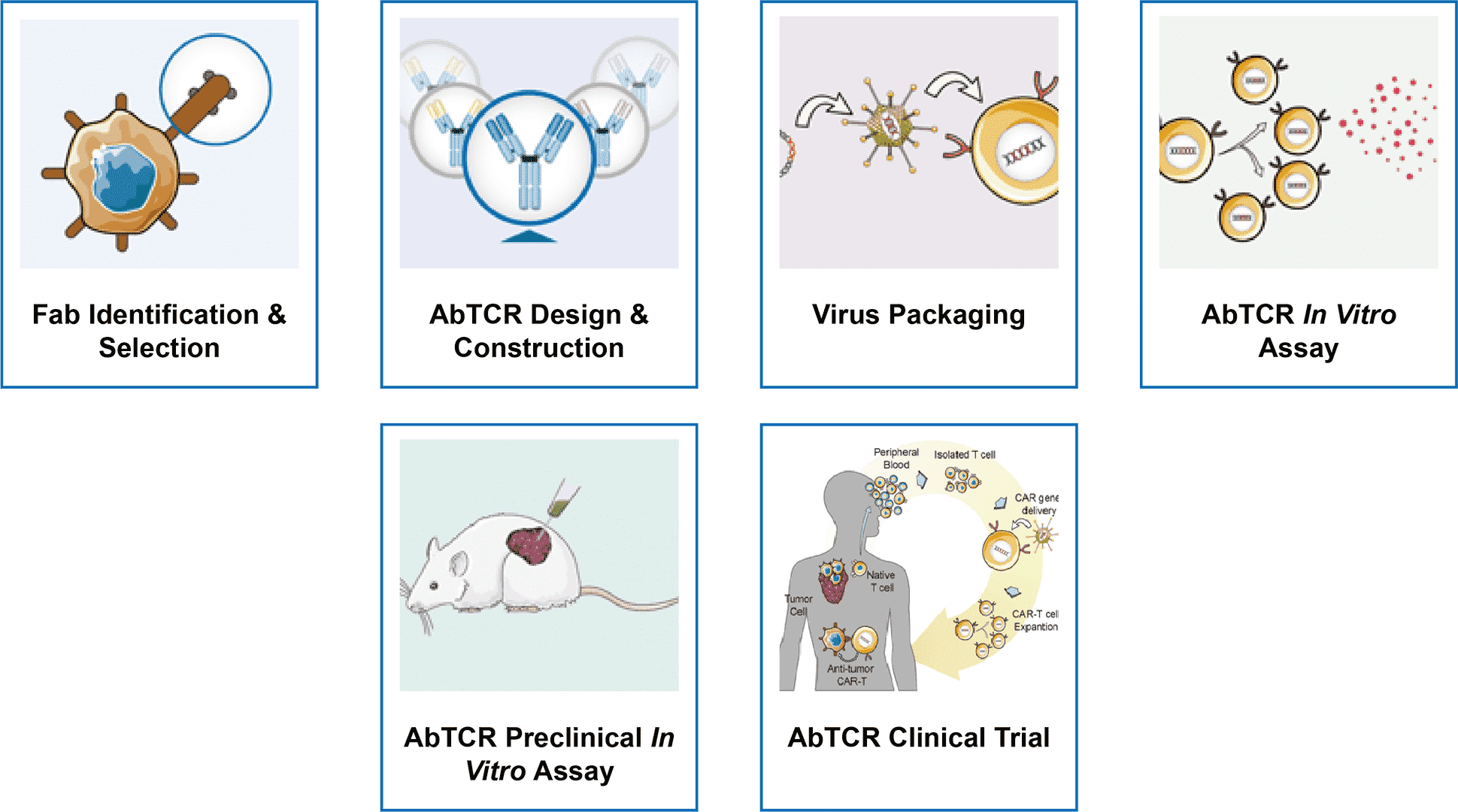
One-stop AbTCR Development Services
Empowered by our high qualified groups and advanced technologies, Creative Biolabs provides one-stop AbTCR development services:
For more detailed information, please feel free to contact us or directly sent us an inquiry.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION