Empowered by first-class platforms and rich experience in the field of antibody discovery and immunotherapy, Creative Biolabs now provides world-leading dendritic cell (DC) vaccine development services as part of DC-based immunotherapy to our worldwide clients. These advanced services are aimed to speed up our clients' projects for academic and clinical purposes.
1. Introduction of DC
Dendritic cells (DCs) exert powerful antigen-presenting functions for the induction of antigen-specific T cell response. Due to this function, DCs are the crucial component of vaccination and DC vaccine has been introduced as a new therapeutic strategy in cancer patients. DC-based immunotherapy is attractive for its safety and efficacy to promote anti-tumor immune responses and prolonged survival of cancer patients. There are a couple of ways to employ DC-based immunotherapy approaches. First, direct targeting/stimulating of the DCs in vivo can be applied to accentuate their anti-cancer phenotype. Second, DCs can be stimulated ex vivo and infused back into the host for carrying out anti-cancer effector functions.
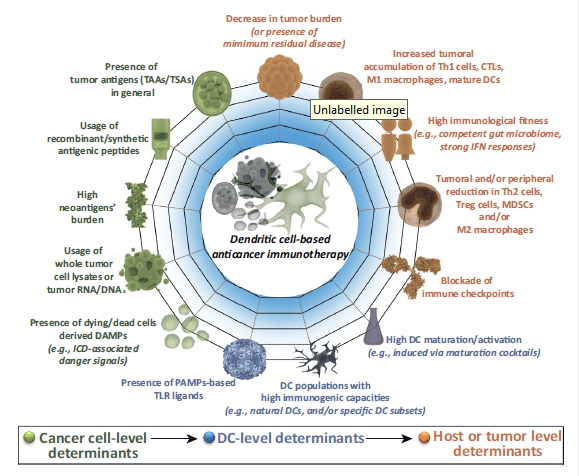
Fig 1 Crucial molecules for anticancer vaccination strategies on the levels of cancer cells, DCs, and the host or tumor. (Garg A D, et al. 2017)
2. Innovated Vaccine Technology
Patient's own immune system can be activated by a cancer vaccine that targets tumor antigens based on the patients'DCs. In this case, effective in vitro activation of DCs is crucial to accurately stimulate the patient's immune system. To meet this demand, Creative Biolabs has discovered a new proprietary method to generate potent DCs and develop DC vaccines based on autologous DCs. These autologous DCs are activated using a proprietary ex vivo maturation process which can be applied for many cancers. In this innovated vaccine technology, the DCs are transiently transfected with mRNA encoding the tumor-specific antigens after activation. More importantly, the construct has been codon optimized and contains a specific targeting sequence resulting in both MHC-I and MHC-II presentation of tumor-specific antigen peptides to both CD8+ and CD4+ T cells in the patients. As a result, this will initiate a strong, tumor-specific immune response after reintroduction into the patient.
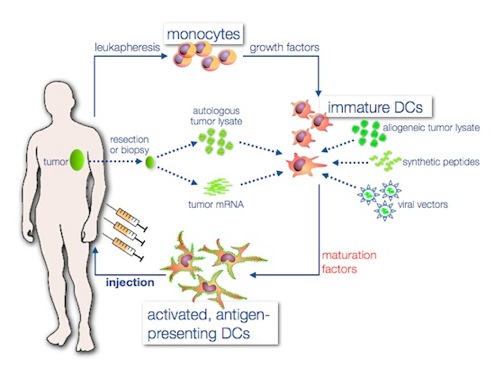
In addition, the popular and promising CAR technology can be used in monocytes, which can be induced and differentiate to DC. These CAR-DC can subsequently contact with tumor antigens. We are pleased to offer this attractive service upon your request.
3. Induction of Antitumor Cytotoxic Lymphocytes Using Engineered Human Primary Blood Dendritic Cells
Leverage the expertise of our experienced scientists to expedite and innovate your immunotherapy projects, we are dedicated to providing engineered human primary blood dendritic cells with the aim of antitumor cytotoxic lymphocytes induction. They can be used to suppress the cancer growth and metastasis. We will help you develop the dendritic cell-based immunotherapy.
4. Highlight Features of Our Services
Over years Creative Biolabs has won good reputation in the field of immunotherapy all over the world. We are more than happy to share our technologies, platforms, and experience to facilitate your projects. We are also confident for our one-stop custom-oriented service packages. Please feel free to contact us for more details and our team will get back to you as soon as possible.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION