Replication-competent virus (RCV) testing is a critical safety requirement in gene therapy development, including CAR-T cell therapy. These tests ensure that viral vectors used in gene therapies are free from replication-capable viruses, which is essential for patient safety and regulatory compliance. At Creative Biolabs, we provide comprehensive RCV testing services with state-of-the-art facilities and extensive expertise in viral vector safety assessment.
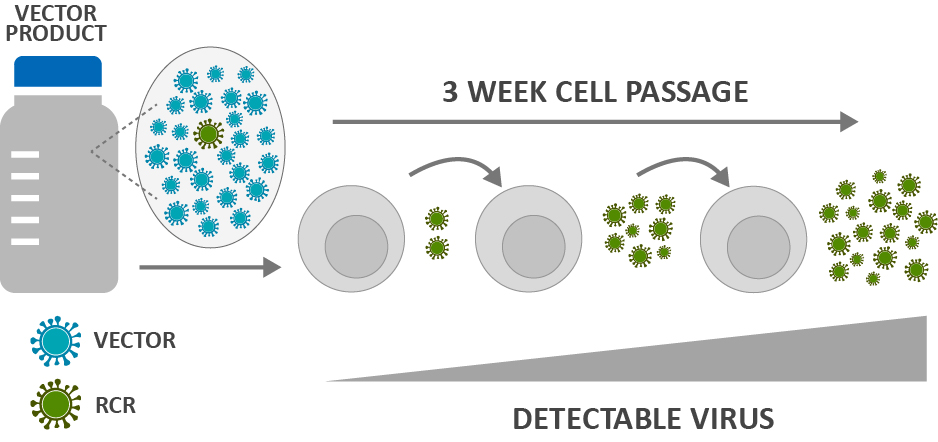
Fig. 1 Biologic assays for replication-competent virus detection.
RCV testing encompasses sophisticated analytical methods designed to detect and quantify replication-competent lentivirus (RCL) or replication-competent retrovirus (RCR) in gene therapy products. The testing process involves multiple complementary techniques, all working together to identify any virus particles that may have regained their ability to replicate independently.
The presence of replication-competent viruses can lead to severe consequences:
Replication-competent viruses can cause serious clinical complications:
Regulatory authorities mandate comprehensive testing throughout development:
Comprehensive safety measures include:
Testing workflow optimization methods include:
| Parameter | Specification |
|---|---|
| Sample Requirements | ≥ 1×10¹³ vg/mL AAV vector |
| Sensitivity | ≤ 10 copies/reaction |
| Parameter | Specification |
|---|---|
| Sample Volume | ≥ 5% of production lot |
| Detection Limit | 1 RCL per 6×10⁸ TU |
| Parameter | Specification |
|---|---|
| Culture Period | 5 passages minimum |
| Detection Sensitivity | 1 RCR per 10⁶ cells |
Our Replication-Competent Virus Testing Services are applicable to various types of samples, including:
The sensitivity of our replication-competent virus testing services depends on the specific detection method employed. qPCR-based methods typically offer high sensitivity, capable of detecting viral genomes at concentrations as low as 10-100 copies/ml. Cell-based assays have slightly lower sensitivity, generally detecting infectious viral particles at levels of 1-10 PFU/ml or 1 TCID50/ml. Protein analysis methods have moderate sensitivity, while the sensitivity of DNA sequencing analysis depends on the sequencing depth, typically detecting low-abundance viral sequences at frequencies of 0.01%-1%. We select the most appropriate testing methods based on our clients' specific requirements, and we can combine multiple methods when necessary to achieve optimal detection performance. We continually validate and improve our testing methods to ensure the highest levels of sensitivity and reliability.
Yes, many of our detection methods can simultaneously detect multiple viruses. qPCR-based methods can utilize multiplex primers and probes or melt curve analysis to differentiate various viruses. Cell-based assays can distinguish multiple viruses by employing different indicator cell lines or observing distinct cytopathic effects. Additionally, high-throughput sequencing technologies enable the simultaneous analysis and identification of all viral sequences present in a sample. We can develop customized multiplex detection methods tailored to the target virus types provided by our clients, fulfilling the need to detect multiple viruses concurrently.
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION