Over the past decade, great progress has been made in the field of immunotherapy, especially in the field of T cell therapy. Chimeric antigen receptor (CAR)-engineered T cell therapy is the most promising approach, which has shown remarkable ability in the elimination of a variety of tumors. While, as more studies focused on B cells, researchers have found that the engineered B cells also have great therapeutic potential in various diseases.
Based on advanced technology and years of research, Creative Biolabs has developed high-quality custom services covering the entire CAR-B therapy development process to best suit customer's technical, program, and budget requirements which can greatly assist customer's research, preclinical investigation, and clinical stage development.
Our one-stop CAR-B therapy development services include but not limited to:
A platform for the immunogenomic reprogramming of hybridoma cells, leading to the rapid generation of cell lines that both surface express and secrete full-length antibodies.
Despite the classical 1st-4th generations of CARs, our Smart™ CAR platform allows to provide construction services of novel CAR designs, such as Dual CAR, TriCAR, Modular CAR, Logic-gated CAR.
We have established a safer approach to cell therapy through the transient expression and non-viral delivery of one or more mRNA molecules into PBMCs or isolated immune cells.
Such methods include knocking out endogenous genes to construct allogeneic universal CAR-T cells, destroying inhibitory receptors and integration of the CAR cassette.
Our CAR-B therapy development services harness the power of two main advantages:
As the best pseudotype candidate, using the LVs pseudotyped with the BaEV envelope to achieve efficient B-cell gene transfer, which can facilitate efficient transduction of resting and stimulated human B cells. This system will be useful in B cell lines, and the range of alternative glycoproteins can be screened for optimal infectivity.
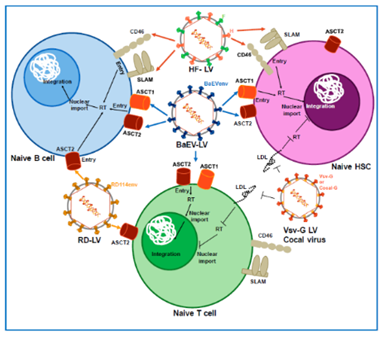 Fig 1. Pseudotyping of lentiviral vectors.1
Fig 1. Pseudotyping of lentiviral vectors.1
CRISPR/Cas9 system has been widely used in recent years due to its advantages of high specificity, high activity, and simple design. The CRISPR design algorithms will be used to develop effective knock-out strategies to remove endogenous BCR (B cell receptor) expression from a B cell line.
Creative Biolabs' scientists are dedicated to bringing together years of valuable experience to help our clients shorten the clinical study journey. We are committed to providing CAR-B development services to reduce the overall project development timeline for our clients. For further details, please don't hesitate to contact us and see how we can help you reach your clinical vision.
Use the resources in our library to help you understand your options and make critical decisions for your study.
Chimeric antigen receptor (CAR) can combine the extracellular antigen recognition domain from antibodies with the immune cell signaling domain to redirect T cell specificity and induce potent antitumor activity. CAR is an artificial transmembrane receptor that connects the extracellular antigen recognition domain, hinge domain (HD), transmembrane domain (TMD), and intracellular signal transduction domain in series.
B cells are lymphocytes essential for the humoral immune response, secreting antibodies to clear foreign antigens. They originate from hematopoietic stem cells in the bone marrow and differentiate into mature B cells through stages involving VDJ recombination for diverse B cell receptors. Mature B cells migrate to peripheral lymphoid organs, where they encounter antigens and receive help from T follicular helper cells (Tfh) to enter germinal centers. There, they undergo somatic hypermutation and class switch recombination, enhancing antibody affinity and function, and differentiate into plasma cells or memory B cells. B cells can also develop in the meninges, highlighting their role in neuroimmune regulation. B cell-mediated immune responses include T-cell-dependent and T-cell-independent mechanisms. B cell-based immunotherapies, such as monoclonal antibodies targeting B cells and adoptive transfer of modified B cells, offer promising cancer treatments by enhancing or modulating immune responses.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION