End-to-End Cell Therapy Development: From Concept to IND
Your trusted CRO partner for integrated solutions. We streamline your path to the clinic with expert services from target discovery and CAR/TCR engineering to preclinical studies.
Our Core Service Areas
We offer a comprehensive suite of cell therapy development services, spanning established technologies to pioneering research.
Oncology Solutions
CAR-T Cell Development
Full service from antigen screening, construct design, viral production, to IND-enabling studies for blood cancers.
CAR-T for Solid Tumors
Overcoming the complex challenges of solid tumors with sophisticated solutions to enhance T-cell efficacy.
CAR-NK Cell Development
Expertise in natural killer (NK) cell sourcing, non-viral editing, expansion, and functional analysis.
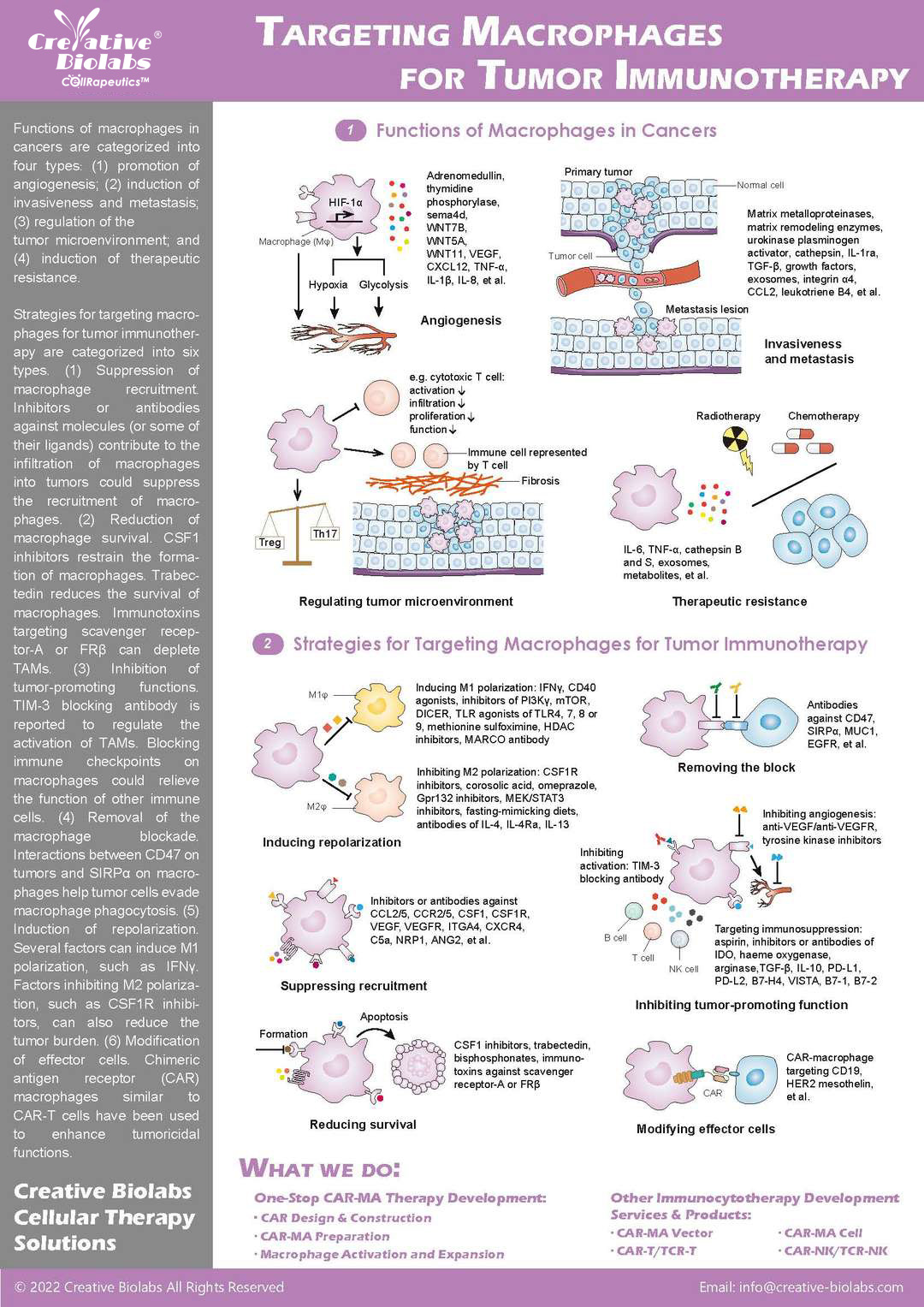
CAR-Macrophage Development
Specialized platforms for CAR-MA, including vector development and phagocytosis assays.
CAR-Monocyte Development
Customized process development for CAR-Monocyte therapies, focusing on myeloid cell advantages.
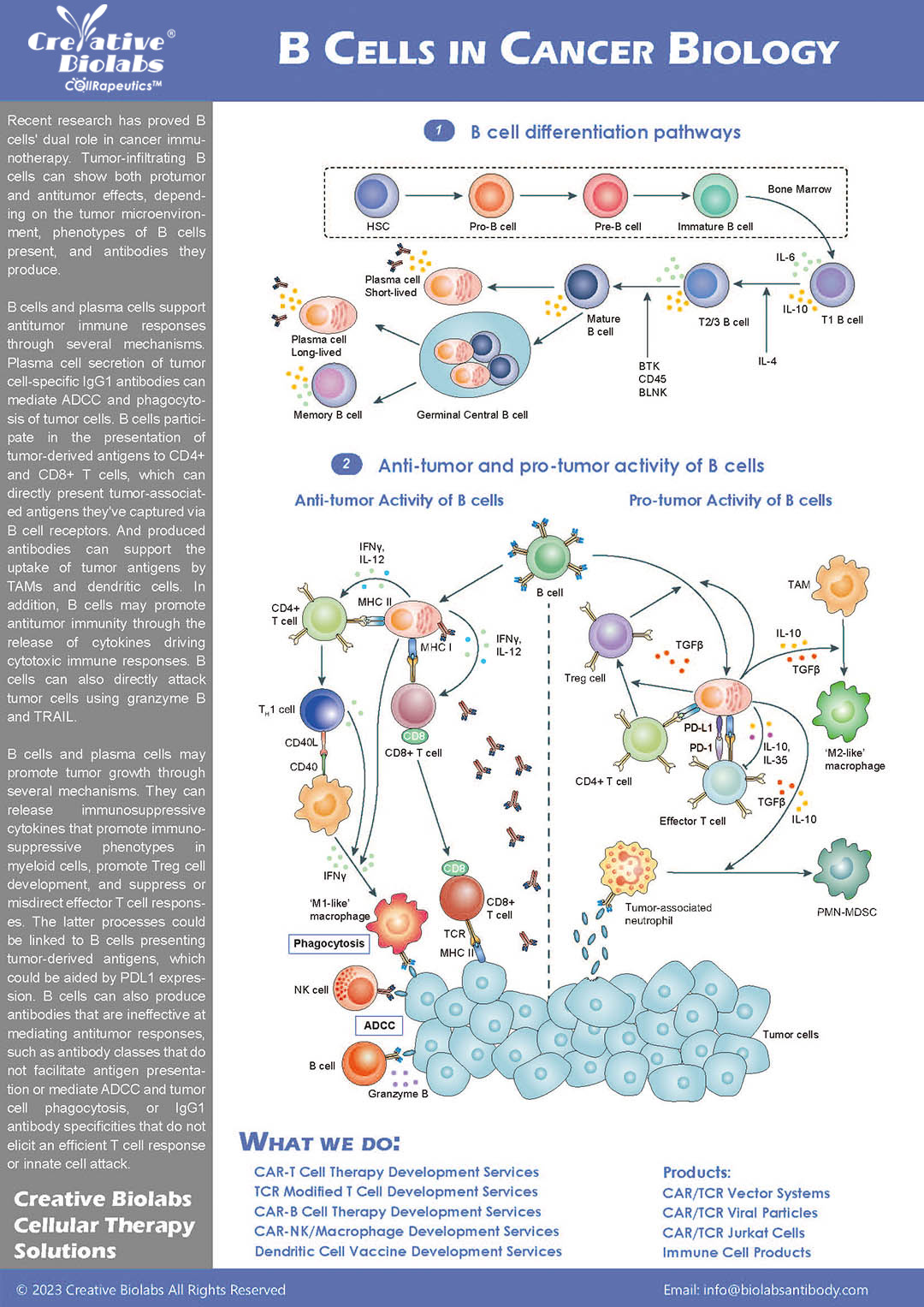
CAR-B Cell Development
Development of CAR-B cells as living drugs, leveraging their unique antigen presentation capabilities.
Emerging Fields & Next-Gen
CAR-T in Non-Cancer Fields
Expanding CAR-T applications to autoimmune diseases, fibrosis, anti-aging, and infectious diseases.
Allogeneic Cell Therapy
Development of "off-the-shelf" therapies using universal sources (iPSC) and gene editing.
In Vivo CAR-T Development
Development of in vivo delivery systems (LNP, AAV) for direct CAR therapy administration.
Specialized Immune Therapies
TCR-T Cell Development
Includes neoantigen screening, high-affinity TCR discovery, and safety/efficacy evaluation.
TIL Therapy Development
Efficient TIL isolation, expansion, and clinical-grade process development from tumor tissues.
CIK Cell Therapy Development
Mature protocols for cytokine-induced killer (CIK) induction and expansion with robust QC.
CAR-Treg Development
Specialized services for CAR-modified regulatory T cell (CAR-Treg) therapies.
Precision DC Vaccine
Utilizing advanced dendritic cell engineering to elicit potent anti-tumor immune responses.
Cancer Vaccine Development
Design of personalized and off-the-shelf cancer vaccines with immune response monitoring.
Enabling Technologies
Functional CAR-T Discovery
Bridging construct and clinic by focusing on cytotoxicity, persistence, and exhaustion resistance.
Metabolic Enhanced CAR-T
Engineering cell metabolism to enhance CAR-T persistence and function in the tumor microenvironment.
Next-Gen Toxicity Management
Advanced solutions to manage CRS & ICANS via logic-gated CARs, suicide genes, and on/off switches.
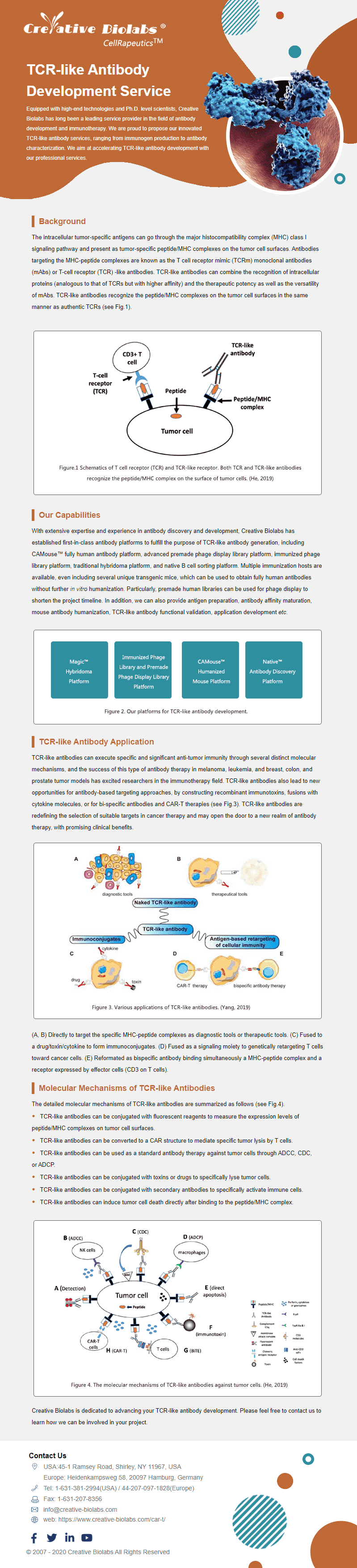
TCR-Like Antibody Development
Development of antibodies targeting peptide-MHC complexes for therapeutic or diagnostic use.
Immune Genotyping
Comprehensive immune profiling to support patient selection and MOA studies.
Artificial T-Cell Stimulation
Custom acellular platforms for robust and consistent T-cell activation and expansion.
Our Proprietary Technology Engine
Our proprietary and cutting-edge platforms empower next-generation cell therapy discovery and development.
CAR × TCR Bispecific T Cell Engineering Platform
This innovative platform integrates both CAR and TCR targeting mechanisms into a single T cell, enabling dual-antigen recognition to enhance tumor-killing specificity and overcome antigen escape.
Surfarray™ Cell Microarray Technology
A high-throughput screening tool that immobilizes thousands of unique cell populations on a single slide, allowing for rapid and parallel functional analysis of cell-cell interactions and antibody binding.
T-Scan Platform for T Cell-Specific Epitope Identification
A genome-wide, high-throughput screening platform that identifies the natural targets of T cell receptors (TCRs) by systematically testing against a comprehensive library of human antigens.
Gamma Delta (γδ) T Cell Platform
Focuses on the unique biology of γδ T cells, which recognize stress-induced ligands on cancer cells in an MHC-independent manner, offering a promising "off-the-shelf" allogeneic therapy option.
Tetravalent Bispecific (TetraBi) T Cell Engager Platform
Develops antibody-based constructs with four binding sites that effectively bridge T cells and tumor cells, inducing potent and specific tumor lysis with improved safety profiles.
CAR-T Cell Display Platform
A powerful tool for antibody discovery and engineering, where functional CARs are expressed on the T cell surface, allowing for direct screening and selection of optimal binders based on functional outcomes.
CAR-Stem Cell Platform
Engineers hematopoietic stem cells with CAR constructs, creating a self-renewing source of cancer-fighting immune cells for long-term, durable therapeutic effects.
CRISPR-edited CAR Cell Technology
Utilizes precise CRISPR/Cas9 gene editing to knock out inhibitory genes (like PD-1) or knock in CAR constructs at specific genomic loci, enhancing the persistence and efficacy of cell therapies.
mRNA-based CAR Cell Platform
A non-viral approach that uses mRNA to transiently express CARs in T cells, offering a safer, "hit-and-run" therapeutic strategy with controllable duration of activity.
Tseeking™ Platform For TCR/Ligand Discovery
An advanced discovery engine that combines cellular immunology and bioinformatics to identify novel, high-affinity TCRs and their specific peptide-MHC targets for therapeutic development.
A Streamlined Path from Concept to Clinic
Our integrated platform ensures seamless transitions between development stages, accelerating your timeline.
1. Discovery & Validation
Identifying novel targets and validating potential binders (scFv, VHH) to ensure high specificity and affinity.
2. Lead Optimization
Engineering and constructing optimal CAR designs, followed by robust in vitro assays to select the most potent candidates.
3. Preclinical Development
Conducting comprehensive in vivo studies in relevant animal models to assess efficacy, safety, and biodistribution.
4. CMC & Manufacturing
Developing scalable and reproducible manufacturing processes for viral vectors and final cell products under stringent quality control.
5. IND-Enabling & Filing
Compiling a complete data package with GLP-compliant studies to support your Investigational New Drug (IND) application.
Why Partner With Us?
We integrate cutting-edge technology with deep scientific expertise to provide true end-to-end solutions, transforming your vision into clinical reality.
Comprehensive Service Portfolio
From CAR-T, CAR-NK, and TCR-T to next-generation allogeneic therapies, our one-stop platform covers the entire development lifecycle. We provide integrated solutions from initial discovery and functional validation to IND-enabling studies, streamlining your path to the clinic.
Cutting-Edge Technology
Leverage our proprietary platforms like T-Scan™ for epitope identification and Surfarray™ for high-throughput screening. Our advanced technologies, including bispecific engineering and mRNA-based CARs, ensure your project stays at the forefront of cell therapy innovation.
Unmatched Scientific Expertise
Our expertise spans a wide array of immune cells, including T-cells, NK cells, Macrophages, and Tregs. We specialize in complex challenges such as enhancing metabolic fitness, managing CRS, and developing logic-gated CARs for improved safety and efficacy, particularly in solid tumors.
Focus on Clinical Translation
We are dedicated to translational success. Our services include robust functional validation, humanized in vivo models, and GLP-aligned data packages to support your IND filings. We provide the rigorous data and mechanistic insights needed to move your candidate from the lab to life-saving therapy.
Trusted by Global Leaders
Explore Our Comprehensive Product Portfolio
High-quality reagents, advanced vectors, and custom products to support and streamline your research workflow.

Can't Find Your Product?
Create a Custom One.
We developed a proprietary online system for customizing CAR and CAR cell products to meet all unique needs with just a few simple clicks.
Try Our Custom SystemFrequently Asked Questions
Find answers to common questions about our cell therapy development services.
We have a novel target. Can you provide a full suite of services from antibody/binder discovery to CAR construction? What is the process?
Yes. We provide a complete development process for novel targets. We start by using phage display libraries, hybridoma technology, or our proprietary Surfarray™ cell microarray technology to screen and discover high-affinity, high-specificity binders (e.g., scFv) for your target. After obtaining candidate binders, we perform affinity measurement and functional validation. Once the optimal binder is selected, our team immediately proceeds with CAR design and construction, and can integrate various optimization strategies (such as optimizing co-stimulatory domains or adding safety switches) before moving into the subsequent functional testing phase.
What are the deliverables upon completion of the preclinical studies? Is this package sufficient to support an IND filing?
Upon project completion, we provide a full, detailed, and comprehensive study report. This report includes all experimental designs, raw data, analysis results, and conclusions, covering everything from CAR construction and in vitro functional validation to in vivo efficacy and safety assessments. For key IND-enabling studies (such as toxicology), we can perform them under GLP (Good Laboratory Practice) compliant conditions and provide reports in a format that meets regulatory requirements. Our goal is to deliver a high-quality, data-rich package to strongly support your Investigational New Drug (IND) application.
During the preclinical in vivo study phase, what animal models can you provide? What do the study endpoints include?
We provide a variety of clinically relevant animal models to support your preclinical studies. These include standard subcutaneous xenograft models, orthotopic models that better mimic the native tumor environment, and patient-derived xenograft (PDX) models that most accurately reflect patient tumor heterogeneity. The study endpoints are comprehensive and typically include: tumor growth inhibition (volume and weight), animal survival analysis, in vivo persistence and biodistribution of CAR-T cells, histopathological analysis of key organs for toxicity assessment, and monitoring for potential side effects such as Cytokine Release Syndrome (CRS).
How is intellectual property (IP) handled?
Our policy is straightforward and client-centric: you retain full ownership of all intellectual property generated from your project. As a pure fee-for-service CRO, our business is built on providing expertise, not on claiming rights to your discoveries. This is formalized through robust legal agreements, beginning with a Confidentiality Disclosure Agreement (CDA). Subsequently, our Master Service Agreement (MSA) explicitly states that all foreground IP—including novel constructs, data, and protocols developed for your therapeutic—is your sole and exclusive property. We ensure a complete IP transfer, giving you the freedom to advance your candidate without future encumbrances from us.
Can I use just one specific service instead of the full package?
Absolutely. While our end-to-end solution offers a seamlessly integrated and accelerated timeline, our services are entirely modular to offer maximum flexibility. We recognize clients have diverse needs at various stages, so you can engage us for a single, specific service or a custom-built package. For instance, you might only require our preclinical in vivo testing using specialized PDX models for an existing construct, or perhaps just our binder discovery services for a novel target. Our team will work closely with you to create a work plan that precisely fills the gaps in your program, allowing you to leverage our specialized expertise exactly where it's needed most.
Related Resources
Dive into our latest webinars, podcasts, and expert articles designed to provide valuable insights for your CAR-T program.
Webinars
Infographics
Flyers
Support Knowledge
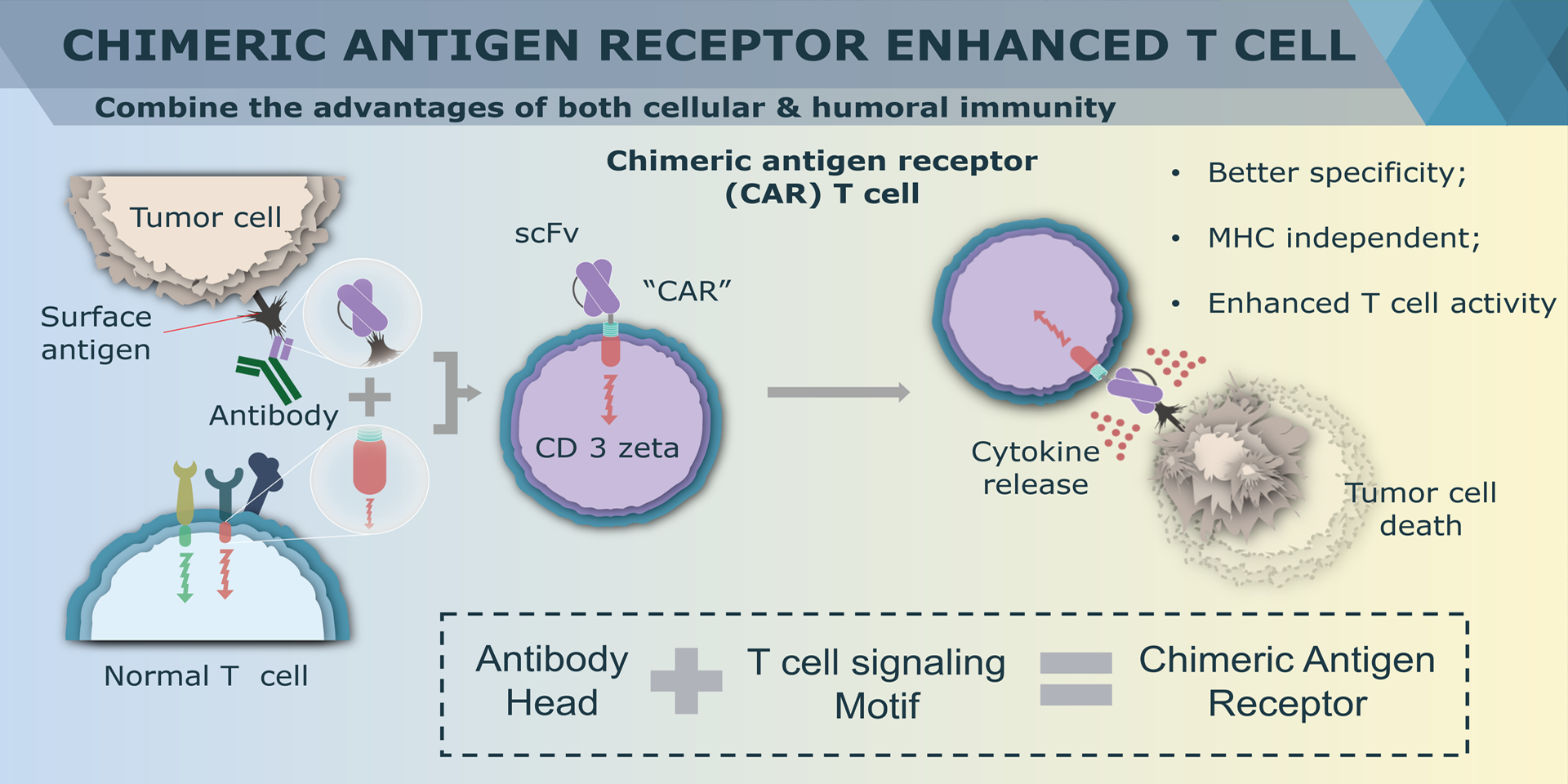
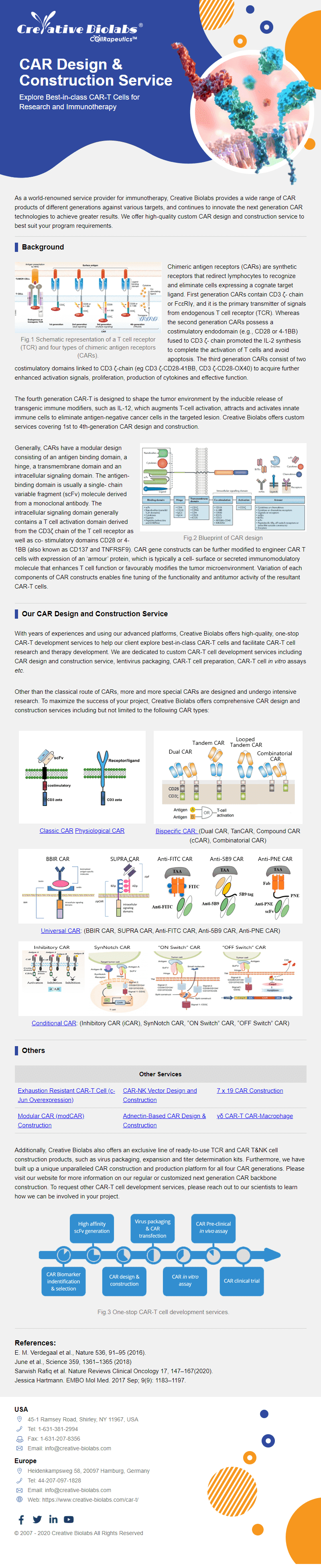
Overview of Chimeric Antigen Receptors
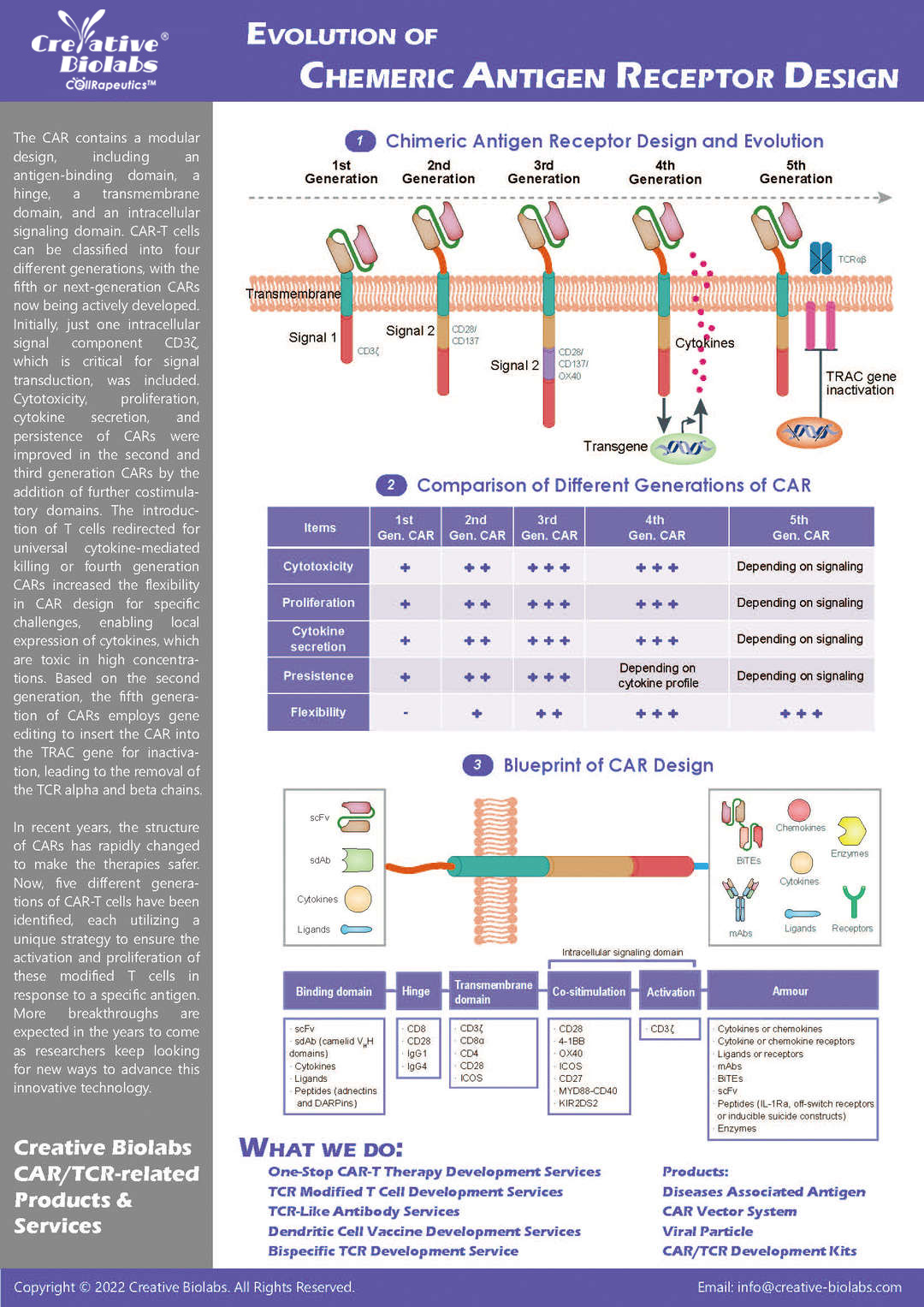
Chimeric antigen receptor (CAR) can combine the extracellular antigen recognition domain from antibodies with the immune cell signaling domain to redirect T cell specificity and induce potent antitumor activity. CAR is an artificial transmembrane receptor that connects the extracellular antigen recognition domain, hinge domain (HD), transmembrane domain (TMD), and intracellular signal transduction domain in series.
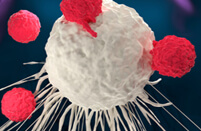
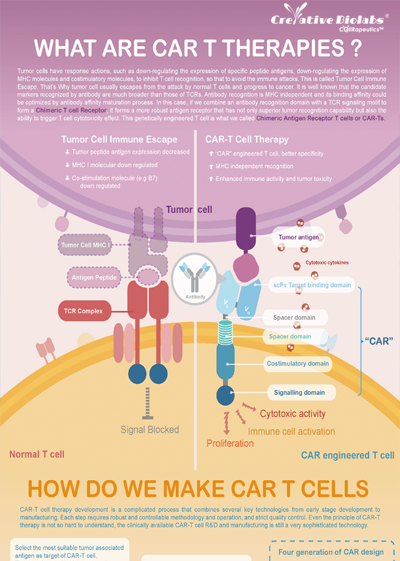
Overview of CAR-T Cell Therapies
CAR-T cell therapies revolutionize cancer treatment by harnessing the power of a patient's immune cells. Engineered with chimeric antigen receptors (CARs), these cells effectively target and destroy cancer cells, offering a personalized and potent approach. This groundbreaking immunotherapy has shown remarkable success in treating certain blood cancers, providing hope for patients who may not respond to traditional treatments. CAR-T cell therapies mark a significant stride towards precision medicine, ushering in a new era in oncology with the potential to transform the landscape of cancer care.
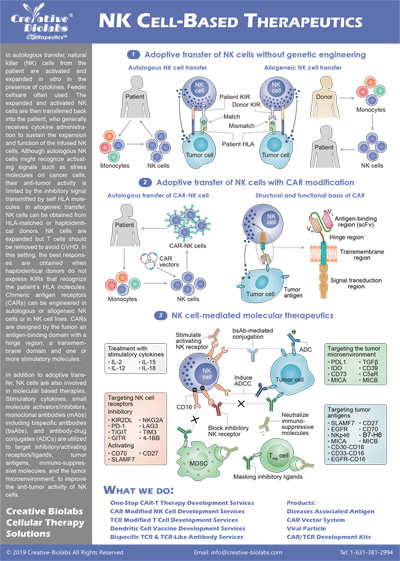
T Cell-based Immunotherapies
Based on the significant roles of T cells in the immune system, many small-molecule drugs targeting T cells and T-cell based immunotherapies have been developed for the treatment of intractable diseases including autoimmune diseases and cancer. T cell-based immunotherapies mainly utilize the mechanisms of T cell-mediated immune responses and the effects of some other immune cells such as dendritic cells (DCs), natural killer (NK) cells, and macrophages.
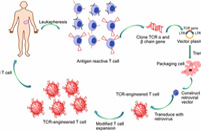
Adoptive Transfer of Engineered T Cells
Adoptive cell transfer (ACT) of engineered T cells is a cutting-edge therapeutic approach revolutionizing cancer treatment. This innovative method involves modifying T cells, a key component of the immune system, to enhance their ability to target and eliminate cancer cells. By introducing genetically engineered T cells into patients, researchers aim to bolster the immune response against cancer, offering a personalized and potentially curative treatment option. This groundbreaking technology holds promise for addressing various malignancies and represents a significant stride towards more effective and precise cancer therapies.
Let's Build the Future of Medicine, Together.
Ready to accelerate your cell therapy program? Contact our experts today to discuss your project needs and discover how we can help you achieve your milestones.