Chimeric antigen receptor (CAR)-engineered T-cell therapy is one of the most promising areas of cancer immunotherapy that has delivered impressive and promising results in the treatment of hematological cancer. With leading expertise and state-of-the-art technology in immunology, Creative Biolabs has established CellRapeutics™ CAR-Technology platform to design improved CAR T-cells for successful tumor elimination while also limiting the development of serious adverse effects.
Though CAR-T therapies have been approved for blood cancers, their application to solid tumors has proved challenging. Preclinical evaluation of CAR-T cell therapy is highly needed. Our scientists have developed a cutting-edge animal model platform to significantly improve the evaluation of CAR-T cell therapy. We are proficient at performing a wide range of preclinical in vivo assays, hoping to answer all your questions through our series of translation models.
Testing CAR T-cells for their suitability in the clinic implies the use of immuno-compromised mouse models. Currently, Creative Biolabs mainly provides two research-ready mouse models, CD34+ HSC Hu-NOD/SCID/IL-2Rγnull Mouse Model and PBMC Hu-NOD/SCID/IL-2Rγnull Mouse Model, which represent two commonly used methods for generating humanized models. NOD/SCID/IL-2Rγnull engrafted with human CD34+ hematopoietic stem cells (HSC) can develop multi-lineage human immune cells, and is effective in vivo model for the evaluation of CAR-T therapy research, other immunology research, and infectious disease research. While PBMC Hu-NOD/SCID/IL-2Rγnull mouse model is generated by injecting human peripheral blood mononuclear cells (PBMC) into female NOD/SCID/IL-2Rγnull mice, and this model is very suitable for short-term studies that require powerful effectors and memory T cells and NK cell functions.
PDX models have been increasingly used in translational research since their development. Studies have shown that PDX models can produce samples that are authentic to the host tumor, and they can accurately replicate tumor growth, diversity of tumor cells, and tumor progression, including metastatic potential. Creative Biolabs has a highly experienced team of scientists who have a long history of successful implementation of PDX model generation and PDX models for the evaluation of CAR-T therapy.
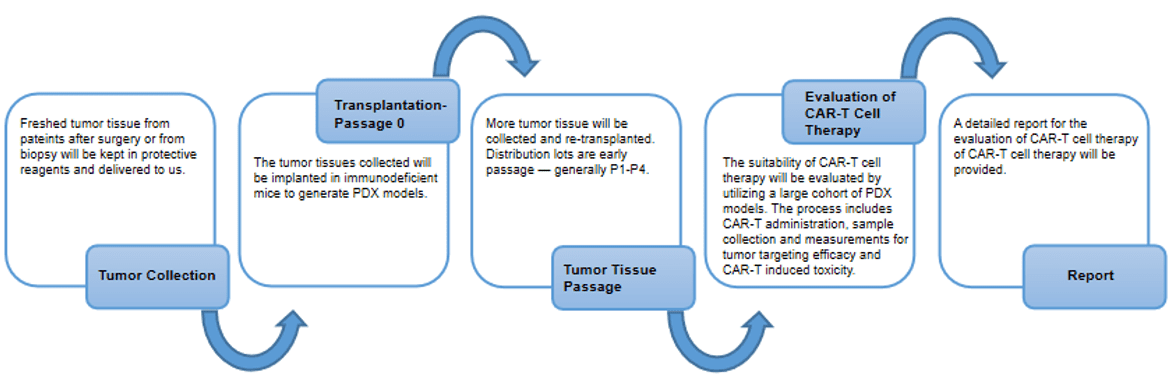
Creative Biolabs has collaborated with hospital and researchers to establishes a central repository of >1700 PDX models of various tumors, including lung cancer, colorectal cancer, gastric cancer, pancreatic cancer, breast cancer, ovarian cancer, head & neck cancer, cervical cancer, esophageal cancer, mesothelioma, and metastatic cancer, etc. We also provide high-quality services for the use of PDX models.
Creative Biolabs has organized a staff of outstanding scientists who have engaged in the preclinical study for CAR-T therapy. Our technologies aim to facilitate the preclinical study for CAR-T therapy research, other immunology research, and infectious disease research. If you are interested in our animal model platform, please contact us for more information and a detailed quote.
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION