Equipped with state-of-the-art facilities and experienced immunology experts, Creative Biolabs is dedicated to providing high-quality GPLA genotyping services for our global clients.
 Distributed under CC BY 3.0, without modification, from Wiki.
Distributed under CC BY 3.0, without modification, from Wiki.
The guinea pig MHC or guinea pig leucocyte antigen complex (GPLA) has been reported since 1973 and is classified into two classes. Class I antigens (BI, BL B3, or B4), these ubiquitous antigens correspond to mouse H2-D and H2-K antigens or to human HLA-A or HLA-B antigens. Class II and associated antigens (Ia), which have been defined as seven specificities (1, 2, 3, 4, 5, 6, 7). The GPLA antigens have a wide tissue distribution and are found on both lymphoid and nonlymphoid cells. In addition, class I and class II antigens are also present on some tumor cells including the L2C-N Leukemia. It was reported that, unlike mice or humans, most normally resting guinea pig T cells and most thymocytes have been reported to express Ia antigens. Class II antigens have also been described on several glandular epithelial cells, including guinea pig mammary gland cells.
With the advancement of genotyping approaches, GPLA genotyping has become accessible. Creative Biolabs has developed a dedicated NGS-based GPLA genotyping service to meet your program requirements. We are committed to providing more comprehensive and targeted accurate genotyping analysis results and facilitating scientific research. Our scientists and technicians are experienced in every step with high dedication to ensuring accurate outcomes.
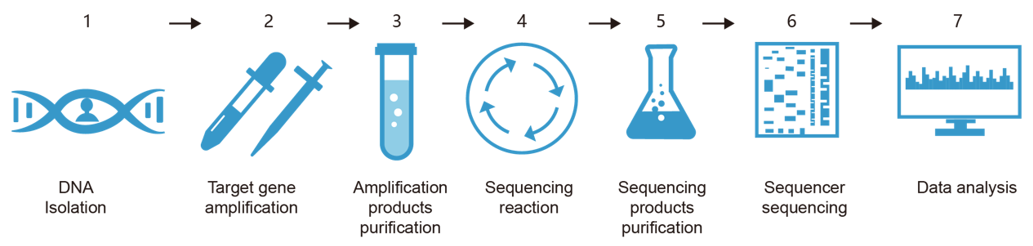 Fig.1 Flow diagram of GPLA genotyping.
Fig.1 Flow diagram of GPLA genotyping.
Creative Biolabs' scientists are dedicated to bringing together years of valuable experience to help our clients shorten the clinical study journey. We are committed to reducing the overall project development timeline for our clients. For further details, please don't hesitate to contact us and see how we can help you reach your clinical vision.
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION