CAR-T (Chimeric Antigen Receptor T-cell) therapy has transformed how we treat hematological malignancies, and its potential for solid tumors keeps growing. But turning a promising CAR-T idea into a viable pre-clinical candidate isn't easy—gene packaging and delivery are the make-or-break steps. Many teams struggle with inconsistent viral vector titers that lead to spotty T-cell transduction, off-target CAR expression that raises safety concerns, or slow scaling that delays timelines. These issues don't just hold back research—they put valuable therapies at risk of never reaching the next stage.
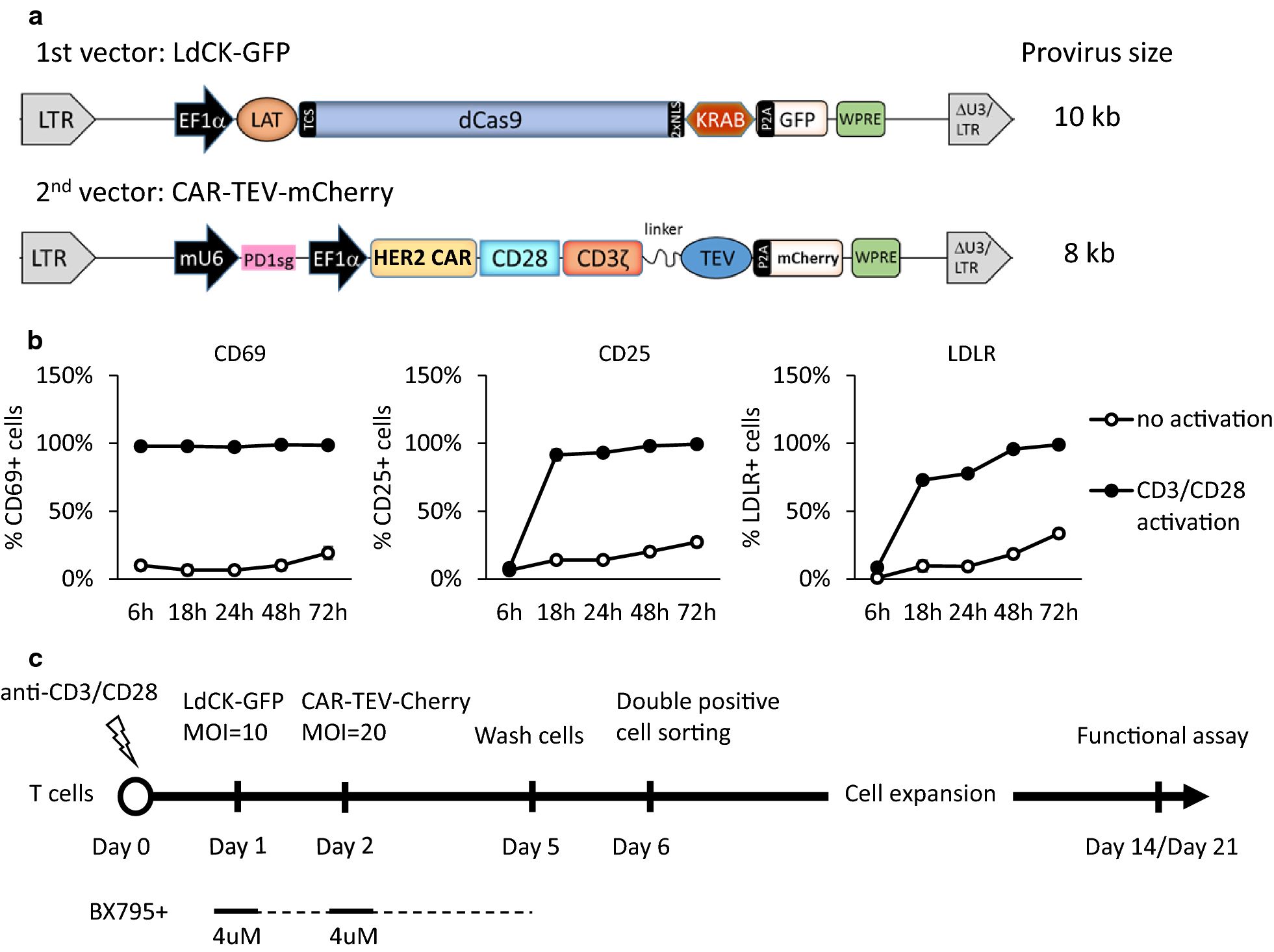 Fig.1 Schematic of lentiviral vector design, T cell activation dynamics, and CAR-T transduction workflow1,2
Fig.1 Schematic of lentiviral vector design, T cell activation dynamics, and CAR-T transduction workflow1,2
You shouldn't have to slow down because of complicated packaging or delivery hurdles. We bring the expertise, tools, and teamwork to help you move faster and avoid common pitfalls.
Our setup grows with your project—no drop in quality. If you start with small batches (like 10⁶ CAR-T cells for in vitro tests to check target binding), we can scale up to large pre-clinical batches (10⁹ cells for in vivo efficacy studies) without changing how we ensure consistency. Whether you need a few vials of vector or a full batch of CAR-T cells, the performance stays the same.
Our scientists (all with PhDs in fields like immunology or viral biology) don't just follow your instructions—they join your team. If you're stuck choosing between a 4-1BB or CD28 costimulatory domain for your CAR, they'll walk you through the pros and cons. If your vector titer was low in past tests, they'll help tweak the design. They proactively share ideas to make your project stronger, not just check boxes.
Safety is built into every step, not added on at the end. We use third-generation lentiviral vectors—these only have three essential HIV-1 genes (gag, pol, rev), so there's almost no risk of making RCVs. Every batch of vector and CAR-T cells goes through strict tests: we check for sterility, measure endotoxin levels, and confirm no RCVs are present. We won't send you anything that doesn't meet pre-clinical standards.
We know you have deadlines—grant submissions, partner meetings, or pre-clinical start dates. That's why every project gets a dedicated manager who keeps you in the loop. They share updates weekly, highlight any potential delays early, and make sure everyone on our team stays on track. You'll never be left waiting to hear if your batch is ready.
Our process is transparent and step-by-step, so you always know what's next and what to expect. Every phase is checked for quality before moving forward.
Collaborative CAR/vector design (suicide gene, reporter integration as needed), codon optimization, and high-purity plasmid DNA production.
Scalable viral vector packaging (293T cells) + purification; T-cell isolation, activation, transduction (optimal MOI), and expansion.
Rigorous testing (flow cytometry, cytotoxicity, sterility); detailed Certificate of Analysis (CoA) and on-time CAR-T cell delivery.
We offer a comprehensive menu of services designed to support your project at any stage, from initial vector design to CAR-T cell generation.
At the core of this workflow, CAR transfection in T cells is our expert service.
Our services use proven, cutting-edge tech to make sure your CAR-T project succeeds.
We've refined our lentiviral vectors to be as safe and effective as possible. We removed all non-essential HIV-1 sequences—only keeping the parts that help the vector package and integrate (LTRs, RRE). This means there's almost no chance of the vector recombining to make RCVs. We also use VSV-G pseudotyping, which makes the vector more stable and lets it transduce different T-cell subsets (even resting ones) —this is a big plus for consistency.
MOI Calibration: We don't just use a "one-size-fits-all" MOI. For every project, we test 3–5 MOIs (from 1 to 10) to find the one that gives >70% CAR expression without killing T cells. This ensures you get functional CAR-T cells, not just high expression.
Cytokine Blends: We use mixes like IL-2 + IL-15 to activate T cells—this doesn't just boost transduction efficiency, it also helps make more memory T cells (which last longer in vivo).
Non-Viral Options: If you don't want to use virus based transfection (e.g., for early research), we offer non-viral methods like electroporation transfection and transposon based transfection —this is good for transient CAR expression.
We give you full transparency so you can trust the products you get from us.
Every shipment comes with a CoA that has all the details you need:
We don't release any product until it passes these checks:
Our services work for all kinds of CAR-T projects, from basic research to pre-clinical tests.
Hematological Malignancy Research
Generate CAR-T cells for leukemia/lymphoma/multiple myeloma (targeting CD19, BCMA, CD22) with long-term CAR expression for extended in vivo mouse model testing.
Solid Tumor CAR-T Development
Support solid tumor studies (neuroblastoma/breast/colorectal cancer, targeting GD2, HER2, CEA) via CAR construct modifications to enhance T-cell tumor penetration and block immunosuppression (e.g., PD-1).
Pre-Clinical Candidate Validation
Produce pre-clinical-grade CAR-T cells/vectors per GLP guidelines, with CoAs containing regulatory-ready data for IND/CTA filings.
Basic Research & Target Validation
Fast delivery of small-batch, high-quality CAR-T cells for early-stage work (new antigen testing, gene function studies, CAR-T-tumor interaction research).
"We fought with low CAR expression for months—our CD19-CAR T cells barely hit 45%, and we couldn't get reliable data for our lymphoma research. Then we worked with Creative Biolabs. They didn't just take our construct—they looked at the sequence, found a codon issue that slowed protein production, and tweaked the signal peptide to get the CAR on the cell surface better. Then they tested 5 different MOIs, found one that got us to 85% CAR-positive cells, and kept T-cell viability over 90%. The CoA they gave was super detailed too—had flow charts, cytokine levels, and how well the cells killed Raji cells. That helped us get a research grant for mouse studies. Now we use them for every CAR-T project—they fix the stuff that holds us up."
Dr. Luisa Mendez
Principal Investigator
"As a small biotech focused on multiple myeloma, we don't have the gear to scale CAR-T production. We needed 10⁶ BCMA-CAR T cells first for lab tests, then 10⁹ cells 6 weeks later for animal studies. Creative Biolabs handled it perfectly. They used the same media, activation mix, and purification steps for both batches—no quality drop. The first small batch was spot-on: 78% CAR expression, almost no endotoxins, no RCVs. The big batch even came 2 days early, and the CD4/CD8 ratio and memory cell count were the same as the small one. Their project manager updated us every week, fixed a minor vector delay in 2 days, and even joined a last-minute call with our partner. No surprises—just solid help."
Ryan Patel
Chief Scientific Officer
"We needed a custom HER2-CAR with a luciferase reporter to track cells in mouse breast cancer studies. Creative Biolabs tested two luciferase types, picked high-brightness luciferase because it's brighter and doesn't mess with T cells. But the first batch had weak signal—they jumped in, did a Western blot, found the luciferase was breaking down, added a tag to stabilize it, and re-made the cells in 5 days. This time the signal was 3x brighter, so we could track CAR-Ts in tumors for 3 weeks. They didn't blame ‘variability'—they fixed it and showed us how. They're not just a vendor—they're on our team."
Dr. Sarah Torres
Director of Cell Therapy
Have questions? Find quick answers here. For more specific inquiries, please don't hesitate to contact us.
What's the difference between lentiviral and retroviral vectors, and how do I pick?
Lentiviral works on both active (dividing) and resting T cells, sticks the CAR gene into the T cell's DNA so it stays expressed long—great if you need CAR-Ts to work in animals for a while. Retroviral only works on dividing T cells (so you need to activate them first) and can't hold as much genetic stuff, but it's faster. Good for short lab experiments where speed matters. We'll help you choose based on your T cells (resting vs. active) and how long you need the CAR to stick around.
Can you work with autologous T-cell samples, and how do you keep them from getting mixed up?
Yep, we do autologous samples (from the patient or model you're studying) all the time. Every sample gets a unique barcode—tracked from when we isolate the T cells to when we expand them. We only process one autologous sample at a time in a dedicated cabinet, and we clean it with UV and chemicals between samples. We also run control tests to make sure no other cells or DNA get in. If you're using donor T cells (allogeneic), we can also help with TCR deletion to lower GVHD risk.
Do you provide regulatory support for IND filings? Do you help with IND/CTA filings, and what does that include?
We do—we follow FDA and EMA rules for pre-clinical tests, and our CoA has all the data regulators need. It includes every step of production, how we tested things (like vector titer or endotoxins), and raw results (flow plots, PCR data for RCVs). Our regulatory team can also check your docs to make sure nothing's missing—we've helped clients get their IND approved on the first try because all the data is clear and follows the rules.
Can you customize the CAR construct—like adding specific parts or safety switches?
For sure. If you want a different costimulatory domain (like OX40 instead of 4-1BB), we'll add it. For safety, we can put in suicide genes (like iCasp9—lets you kill CAR-Ts if there are side effects) or miRNA sites (stops CAR from working in healthy cells). We've also added signal peptides to get the CAR on the cell surface better, or dual reporters (GFP + luciferase) for tracking. Just tell us what you need, and we'll design and test it.
What if I already have a CAR plasmid—can you use it, and what checks do you do?
Absolutely. First, we look at the plasmid map to make sure it has the parts we need (like LTRs and packaging signals). Then we test if it's pure (no degradation) and has low endotoxins—if not, we'll clean it up. We also do a small test run to make sure the plasmid makes working vector and gets the CAR on T cells. If we find a problem (like a missing part), we'll tell you early so we can fix it—no wasted time on failed runs.
References
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION