The vaccine is a biological agent that enhances immunity to a particular disease. Over time, it has evolved from traditional prophylactic uses to more complicated therapeutic products. The cancer vaccine development is a pathway from discovery research to clinical evaluation of putative protective antigens. The process is unique, capital intensive and risky. Given the significance of safety with biologics, the vaccine industry is highly valued.
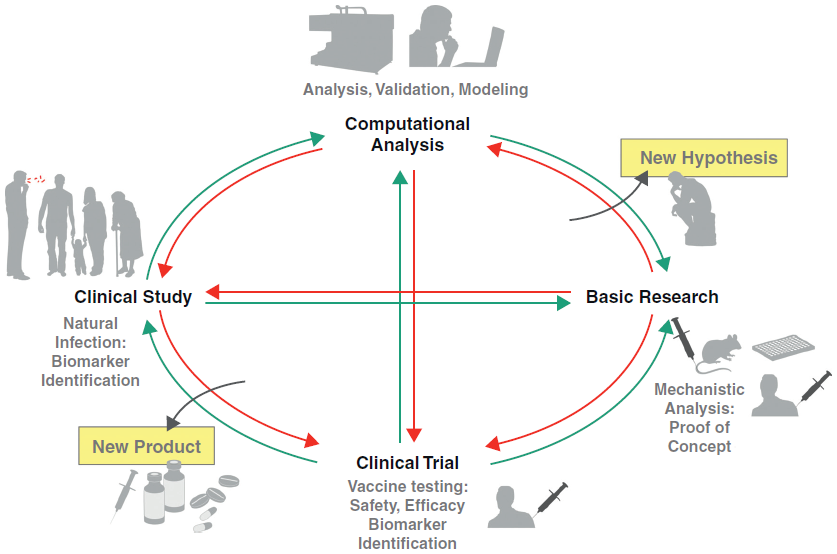
Fig.1 Life cycle of modern vaccine development.1
There are a lot of challenges in the field of cancer vaccine development. it is essential to be in balance with cost, risks, and benefits besides finding safe and effective antigens, adjuvants, and delivery systems. Today, Creative Biolabs makes efforts to meet the steadily growing demands for novel cancer vaccines and accelerate vaccine design, development, and manufacturing through international collaboration and continuous innovation of technologies.
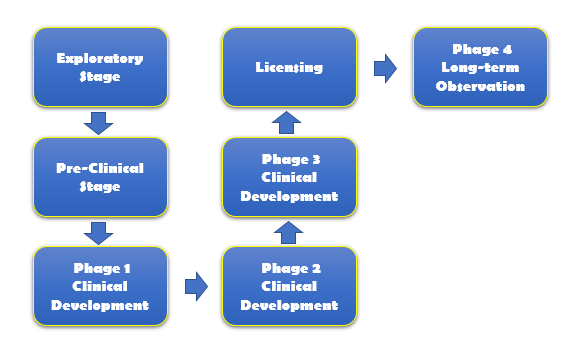 Development of every vaccine starts at the exploratory stage. The goal of therapeutic cancer vaccines is to induce immunity activities against tumor-associated antigens (TAAs), which fall into two categories: tumor-specific shared antigens and tumor-specific unique antigens.
Development of every vaccine starts at the exploratory stage. The goal of therapeutic cancer vaccines is to induce immunity activities against tumor-associated antigens (TAAs), which fall into two categories: tumor-specific shared antigens and tumor-specific unique antigens.
Cancer vaccine developments can be divided into two stages, pre-clinical and clinical development. Pre-clinical studies comprise laboratory and animal experiments. In the clinical stage, the vaccines are tested in humans and this process takes four phases and lasts for many years. Currently, we hope to introduce the following services to allow for a more streamlined and profitable vaccine development.
Creative Biolabs supports cancer vaccine development in both the pre-clinical and clinical phases. Our unique combination of expertise makes us an ideal partner to facilitate clients' vaccine research from the identification of potential targets, analysis of immune responses, to assessment in animal models.
 Cancer vaccines are made in the same way as other biologicals, but with higher standards, to ensure the purity and quality of final products. Every step from the beginning must be confirmed and the whole process of manufacture must be carried out in a clean, safe, and controlled environment.
Cancer vaccines are made in the same way as other biologicals, but with higher standards, to ensure the purity and quality of final products. Every step from the beginning must be confirmed and the whole process of manufacture must be carried out in a clean, safe, and controlled environment.
In Creative Biolabs, cancer vaccines are prepared following Good Manufacturing Practices (GMP) and our cleanrooms are a part of GMP regulations for vaccine development. The circumstance of humidity, air, and temperature in this room are controlled along with the number of entering people simultaneously. According to the requirements, we can meet three GMP standards, including ISO, EU, and US-FDA, and we can deal with different issues in vaccine production.
General GMP concerns for upstream; Raw material preparation; Cell culture; Virus culture; Inactivation of microorganisms.
General GMP concerns for downstream; (ultra) Filtration techniques; (ultra) Centrifugation techniques; Sterile filtration and aseptic processing.
Vaccination remains the most cost-effective public health intervention, whose benefits extremely outweigh the costs. Here, Creative Biolabs can offer multiple products and services in the process development of cancer vaccines, GMP manufacturing, and analytical testing, as well as assist clients in pre-clinical and clinical research to support vaccine commercialization.
For more information on the solutions of vaccine-related services, please contact us by e-mail.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION