Empowered by extensive experience and state-of-the-art equipment, Creative Biolabs has been a world-leading custom manufacturer in the area of antibody discovery and immunotherapy. Now, we are proud to introduce our full package service of investigational new drug (IND) development for chimeric antigen receptor (CAR) T cell therapy.
Cell-based immunotherapies using CAR-T cells have demonstrated high response rates in patients with B cell malignancies. Currently, this therapy is being investigated in both hematologic and solid tumor types. As more and more CAR-T cell therapies aim to move into clinical trials and become promising treatment options for more patients, IND development is required and becomes a topic for extensive discussion. However, it is challenging from the experiment design, project management, to the file preparation of each module based on regulations from Food and Drug Administration (FDA) for most scientists who are not expert at them. Therefore, Creative Biolabs, who has been in the field for years, offers the IND development service for CAR-T cell therapy to solve your troubles during the whole IND process.
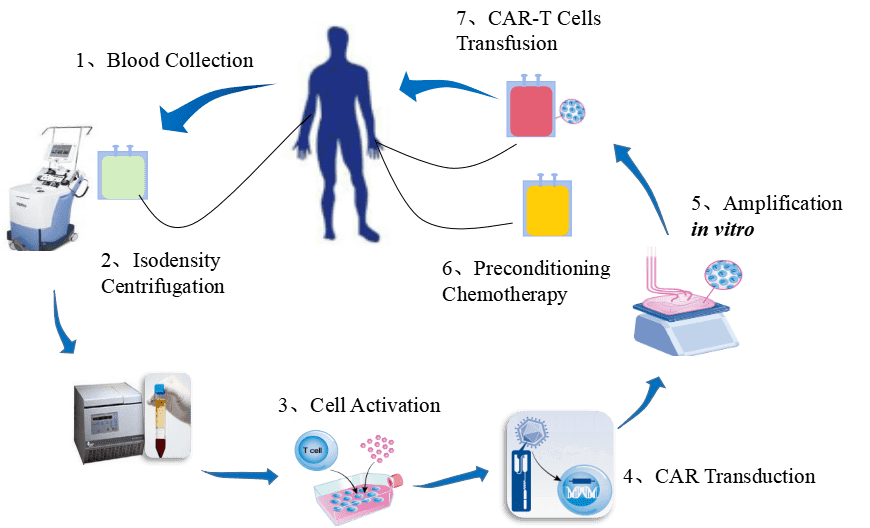
Figure 1. The procedure of CAR-T cell therapy.
As an experienced company, Creative Biolabs deeply understands FDA's current requirements on this item. There are two fundamental purposes for FDA to review an IND. The first one is to ensure the safety and rights of subjects in all phases of an investigation. The second is to help ensure that the quality of the scientific evaluation of the drug is sufficient to permit an evaluation of the drug's safety and effectiveness in phases 2 and 3. A full IND development process includes the pre-IND process, the IND study protocol, preparing the initial IND submission, filing the IND, maintaining the IND. The pre-IND process is served in determining if your study qualifies for exemption from an IND. A fully developed clinical protocol is the basis for the initial IND submission. Preparing the initial IND submission includes an IND application form (contains animal pharmacology and toxicology studies, manufacturing information), a cover letter, a ClinicalTrials.gov certification of compliance. Our one-stop IND development service can free up your valuable time, helping you complete the whole IND process from the pre-IND process to maintaining the IND.
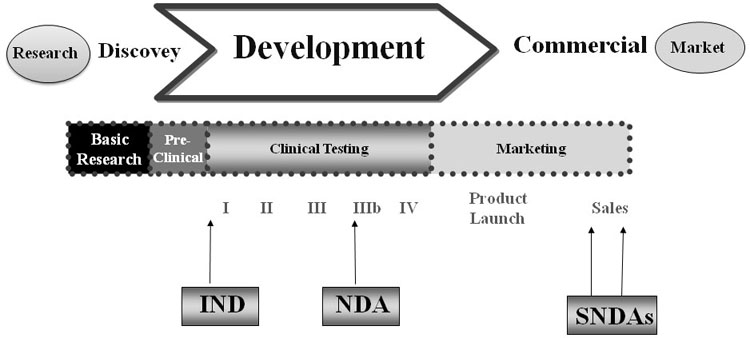
Figure 2. Drug development phases from research stage up to marketing; NDA = New Drug Application, SNDAs = Supplemental New Drug Application.
We are proficient in every process of IND Development. Choosing Creative Biolabs can certainly increase the possibility of passing. if you are interested in our service, please feel free to contact us for more information. For more details, please click CAR-T safety testing.
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION