Powered by our in-house scientists and advanced technological platforms, Creative Biolabs is committed to providing canine CAR-T development service to best suit the client's program requirements.
Compared with other mammals, the canine genome shares greater homology with the human genome, along with a very high degree of similarity in the characteristics of tumorigenesis. Using the characteristics of spontaneous cancer in dogs for CAR-T evaluation will promote the development of CAR-T cell therapy for cancer, and it is expected to improve the evaluation parameters for preclinical CAR-T cell product selection.
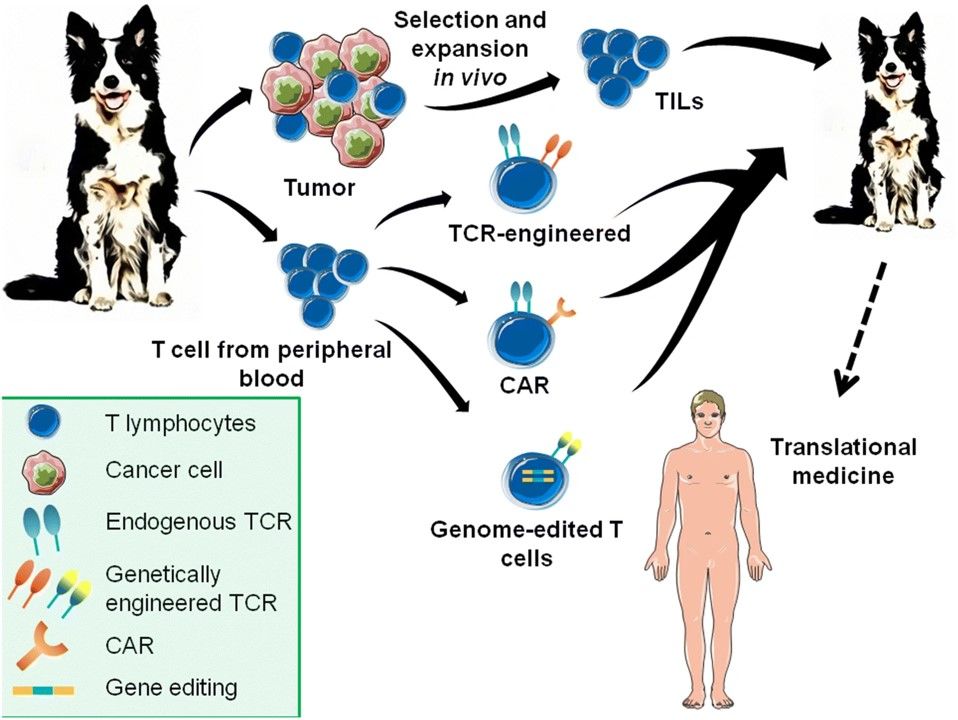 Fig.1 Adoptive cell immunotherapy possibilities for cancer in dogs may facilate new clinical trials for humans.1
Fig.1 Adoptive cell immunotherapy possibilities for cancer in dogs may facilate new clinical trials for humans.1
Hematologic malignancies are a large group of cancers affecting the blood, bone marrow, and lymph nodes, afflicting millions of adults and children every year, and are often fatal. With our years of experience in the CAR-T field, Creative Biolabs is committed to offering canine CAR-T development services for hematologic malignancies. Our service is featured with the following items.
As more and more hematologic malignancies markers have been discovered, we have launched canine CAR-T development services for hematologic malignancies with a lot of popular targets. Here are some popular targets you can choose to design for, including but not limited to:
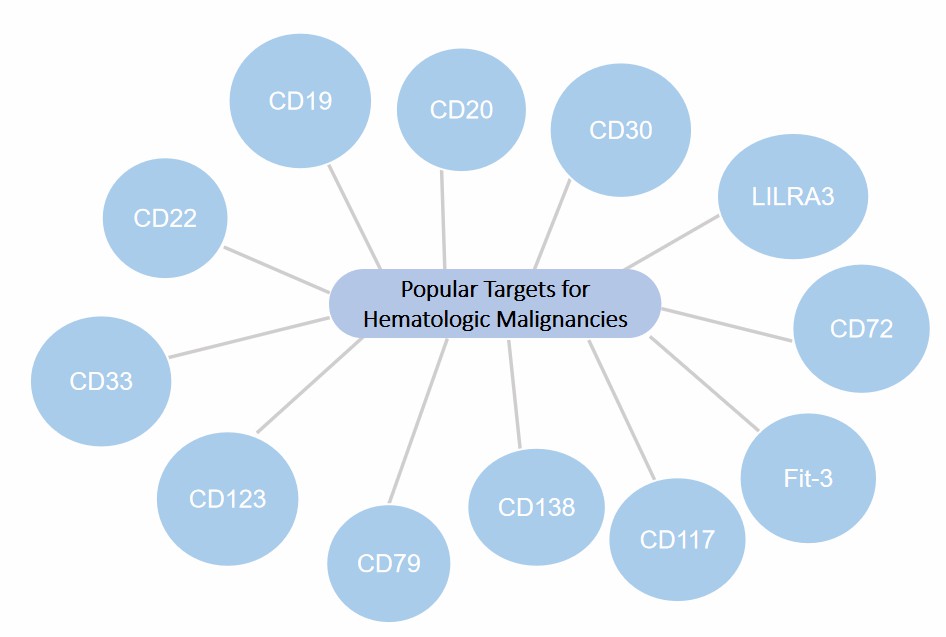 Fig.2 Popular targets profile for hematologic malignancies. (Creative Biolabs)
Fig.2 Popular targets profile for hematologic malignancies. (Creative Biolabs)
For CAR design and construction, we offer classic and special CAR design and construction solutions for clients to reference and choose from. The following are some of our CAR designs, including but not limited to:
We offer the following processes, from target selection and design optimization to in vitro and in vivo functional validation. At the same time, we have a professional technical team to support the optimization and implementation of the project for every step of the process.
 Fig.3 The flowchart of CAR-T development services. (Creative Biolabs)
Fig.3 The flowchart of CAR-T development services. (Creative Biolabs)
For more detailed information about canine CAR-T development targeting hematologic malignancies, please feel free to contact us or directly sent us an inquiry.
Reference
For any technical issues or product/service related questions, please leave your information below. Our team will contact you soon.
All products and services are For Research Use Only and CANNOT be used in the treatment or diagnosis of disease.
 NEWSLETTER
NEWSLETTER
The latest newsletter to introduce the latest breaking information, our site updates, field and other scientific news, important events, and insights from industry leaders
LEARN MORE NEWSLETTER NEW SOLUTION
NEW SOLUTION
CellRapeutics™ In Vivo Cell Engineering: One-stop in vivo T/B/NK cell and macrophage engineering services covering vectors construction to function verification.
LEARN MORE SOLUTION NOVEL TECHNOLOGY
NOVEL TECHNOLOGY
Silence™ CAR-T Cell: A novel platform to enhance CAR-T cell immunotherapy by combining RNAi technology to suppress genes that may impede CAR functionality.
LEARN MORE NOVEL TECHNOLOGY NEW SOLUTION
NEW SOLUTION
Canine CAR-T Therapy Development: From early target discovery, CAR design and construction, cell culture, and transfection, to in vitro and in vivo function validation.
LEARN MORE SOLUTION