Antisense Oligonucleotides (ASOs) Products for HIV/AIDS
Anti sense oligonucleotides (ASO) represents one of the most promising and mature therapeutic approaches over recent years. Consisting of short synthetic nucleic acid fragments, these molecules are able to bind with high specificity to a target messenger RNA (mRNA) or precursor mRNA. This combination then activates a series of molecular processes which regulate gene expression and result in effective silencing of pathogenic protein production. At Creative Biolabs, we focus on the design and development of customized ASO, utilizing their unique features to address the ongoing challenges in HIV/AIDS treatment.
Background
It is well known that HIV/AIDS is a spectrum of conditions caused by infection with HIV. Nowadays, ASOs are under investigation as new drugs used to stop HIV infection. During the cycle life of HIV, antisense drugs can be designed to block the reverse transcriptase and inhibit virus gene expression by antisense specific activity. Prior attempts to inhibit HIV by various antisense approaches have used oligonucleotides targeted to the initiation sites for translation, the cap site, the polyadenylation signal, the RT primer site, the splice donor/acceptor sites, and the site between the gag and pol genes, etc. In addition, it has found that antisense compounds that specifically bind the TAR (trans-acting responsive element) within RNA structure and interfere with tat trans-activation have activity as therapeutic agents for HIV infection.
The Advantages of ASO-Based HIV Therapy
Compared with traditional small molecule drugs, anti-tumor active peptides (ASO) have the following significant advantages:
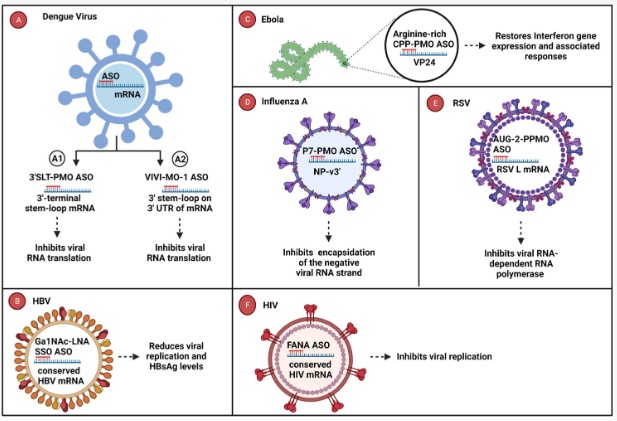 Figure 1. Antiviral oligonucleotides targeting essential viral genes.1
Figure 1. Antiviral oligonucleotides targeting essential viral genes.1
- High specificity: The binding of ASO is based on sequence complementarity, therefore it has high specificity and minimizes off target effects to the greatest extent possible.
- Durability: ASOs that have been chemically modified have a longer half-life in tissues, which can reduce the frequency of administration (e.g. once or twice a year) and significantly improve patient compliance and quality of life.
- Overcoming drug resistance: Due to ASO targeting nucleic acids rather than proteins, they can circumvent the common resistance mechanisms of traditional antiviral drugs.
- Complementary mechanism: The mechanism of action of ASO is different from existing therapies, making it an ideal choice for combination therapy.
How Do Antisense Oligonucleotides Work?
The therapeutic effect of ASO is not limited to a single mechanism; On the contrary, it is a multifunctional platform that can be customized based on specific biological outcomes. The main mechanism of ASO action for HIV is:
-
RNase H-dependent Cleavage
This is a classic and widely studied mechanism. ASOs with thiophosphate backbone and deoxyribose ring can be recognized by RNase H, a widely present cellular enzyme. When ASO binds to its target HIV RNA, it forms a DNA-RNA heteroduplex. RNase H subsequently recognizes the double stranded body and breaks down the RNA strand through enzymatic cleavage, leading to its degradation.
-
Steric Hindrance
ASOs can be designed to bind to target RNA without activating RNase H. On the contrary, their physical presence on RNA strands can hinder their binding to important cellular mechanisms such as ribosomes or splicing factors in space. his prevents translation or alters the processing of the RNA molecule.
-
Splicing Modulation
The HIV genome relies on complex alternative splicing patterns to generate multiple proteins from a single pre mRNA transcript. Antisense oligonucleotides (ASOs) can be designed to regulate this splicing process. By binding to the pre mRNA splicing site, ASOs can promote exon skipping or insertion, thereby altering the final protein product.
How to Design Antisense Oligonucleotides?
Designing effective ASOs is a complex multi-step process that requires in-depth biological insights and advanced bioinformatics techniques. ASOs are typically short single stranded DNA or RNA analog sequences, ranging in length from 15 to 22 nucleotides. The key to its use in HIV/AIDS treatment lies in identifying and targeting key viral RNA sequences. The goal is to create sequences that are complementary to key regions of viral RNA, such as translation start sites, splicing sites, or reverse transcription sites.
Key design considerations include:
- Target Selection
- Sequence Specificity
- Chemical Modifications
- Length
Common Chemical Modifications in ASO Design for HIV Therapy
| Modification Type | Key Properties | Advantages | Challenges |
|---|---|---|---|
| Phosphorothioate (PS) | Nuclease resistance, Protein binding | Extended half-life, Tissue distribution | Coagulation effects, Immunostimulation |
| 2'-O-Methyl (2'-OME) | Increased binding affinity | Nuclease resistance, Reduced immunogenicity | Does not support RNase H cleavage |
| 2'-O-Methoxyethyl (2'-MOE) | Enhanced binding affinity, Nuclease resistance | Favorable pharmacokinetics, Potency | Potential hepatotoxicity at high doses |
| Locked Nucleic Acid (LNA) | Superior binding affinity, High nuclease resistance | High potency, Allows shorter sequences | Potential hepatotoxicity, Requires careful dosing |
| Phosphoroselenoate | Moderate nuclease resistance (t½ ~30 days) | Novel mechanism | Reduced hybridization, Higher toxicity |
Antisense Oligonucleotide Delivery
The biggest challenge faced by antiretroviral (ASO) based therapies is how to efficiently and specifically deliver to target cells, especially within the host immune system, namely HIV host cells. Exposed antiretroviral (ASO) can be rapidly degraded by nucleases in the blood, and due to its polyanionic nature, it is difficult to penetrate the cell membrane. Creative Biolabs offers a variety of delivery solutions to overcome these hurdles:
- Conjugate Chemistry
- Lipid Nanoparticles (LNPs)
- Cell-Penetrating Peptides (CPPs)
Customized Product Process
At Creative Biolabs, our custom ASO product development process for HIV/AIDS is a systematic, customized workflow designed to meet specific research and therapeutic needs, integrating biological insights and advanced technologies:
- Target-to-Need Matching - We first collaborate with clients to identify key targets (e.g., targeting viral replication genes or latent reservoirs) and confirm key parameters, such as the target RNA sequence (e.g., TAR element, gag/pol region) and the desired mechanism (RNase H cleavage or splicing regulation).
- Project Execution - This phase integrates sequence design, in silico screening, and customized chemical modifications. We use bioinformatics tools to design 15-22 nucleotide sequences complementary to the HIV target region and then screen them for specificity and binding affinity. Based on the intended mechanism and delivery route, we optimize modification combinations (e.g., PS + 2'-MOE/LNA) and validate their target compatibility.
- Synthesis and Quality Control - We synthesize high-purity (>95%) ASOs and subject them to stringent quality control, including molecular weight verification by mass spectrometry and purity analysis by high-performance liquid chromatography.
Frequently Asked Questions
Q: What is the difference between ASO and siRNA?
A: Although ASO and small interfering RNA (siRNA) are both nucleic acid based gene silencing drugs, their mechanisms of action are different. ASO is single stranded and can function through RNase H-mediated cleavage or steric hindrance. SiRNA is double stranded and functions as part of RNA induced silencing complex (RISC), which cleaves target mRNA.
Q: How does ASO for treating HIV compare to traditional antiretroviral therapy?
A: Compared with traditional small molecule antiretroviral drugs, ASO has several potential advantages: (1) higher resistance barrier due to targeting conserved genomic regions; (2) Longer duration of action can reduce the frequency of administration; (3) By targeting cytokines or directly acting on latent areas, it is possible to reduce the virus pool; (4) It has a potential new mechanism of action that may be effective against drug-resistant strains. However, antiretroviral vectors (ASOs) face challenges in delivering to viral libraries, requiring special chemical modifications to ensure stability and minimize toxicity.
Q: What are the main toxicity issues of antiretroviral vector (ASO) therapy for HIV?
A: The main toxicity issues include: (1) hybridization dependent off target effects due to partial complementarity with human transcripts; (2) Non hybrid dependent effects, including immune stimulation (such as CpG mediated), coagulation abnormalities, and cumulative toxicity in the kidneys and liver; (3) Injection site reaction of subcutaneous injection of antiretroviral vector (ASO); And (4) potential immune activation that may have adverse effects on the progression of HIV disease. These issues can be addressed through careful sequence design, chemical modifications, and comprehensive safety assessments.
Q: What chemical modifications are most promising for anti HIV and antiretroviral vectors (ASO)?
A: Promising modifications include: (1) thiophosphate bonds for nuclease resistance and protein binding; (2) 2 '- O-methoxyethyl (2'MOE) modification can enhance binding affinity and reduce immunogenicity; (3) Locking nucleic acid (LNA) has excellent binding affinity and potency; (4) Bridge nucleic acid (BNA), including cET and cMOE variants, can provide a balanced efficacy and safety; (5) New modifications, such as selenophosphates (carefully evaluated for toxicity). The optimal modification mode depends on the specific mechanism of action and delivery method.
Drop Us a Line Today!
Creative Biolabs is a world-renowned service provider for antisense oligonucleotides therapy development. We provide a variety of top-quality ASO products for HIV/AIDS therapy. In addition, a series of stringent criteria are applied to implement quality control of antisense products in order to guarantee reliability. We are dedicated to providing the best-characterized antisense therapy service to promote customers' project applications. Please feel free to contact us by e-mail for a quote and further technical support.
Reference
- Buthelezi L A, Pillay S, Ntuli N N, et al. Antisense therapy for infectious diseases. Cells, 2023, 12(16): 2119. https://doi.org/10.3390/cells12162119 (Distributed under Open Access license CC BY 4.0, without modification.)
