Phosphorothioate Modification Service
Introduction
Phosphorothioate (PS) modification, replacing a non-bridging oxygen in the phosphate backbone of oligonucleotide with sulfur, enhances therapeutic oligonucleotides' enzymatic resistance, boosting bioavailability, extending half-life, and enabling gene silencing/editing by addressing instability and off-target issues. Our service leverages this to enhance nucleic acid drug stability and efficacy, improving pharmacokinetics, broadening therapeutic windows, and delivering products with preserved activity, reducing dosing frequency, and improving outcomes via rigorous characterization.
[Discover How We Can Help - Request a Consultation]
Phosphorothioate Modification
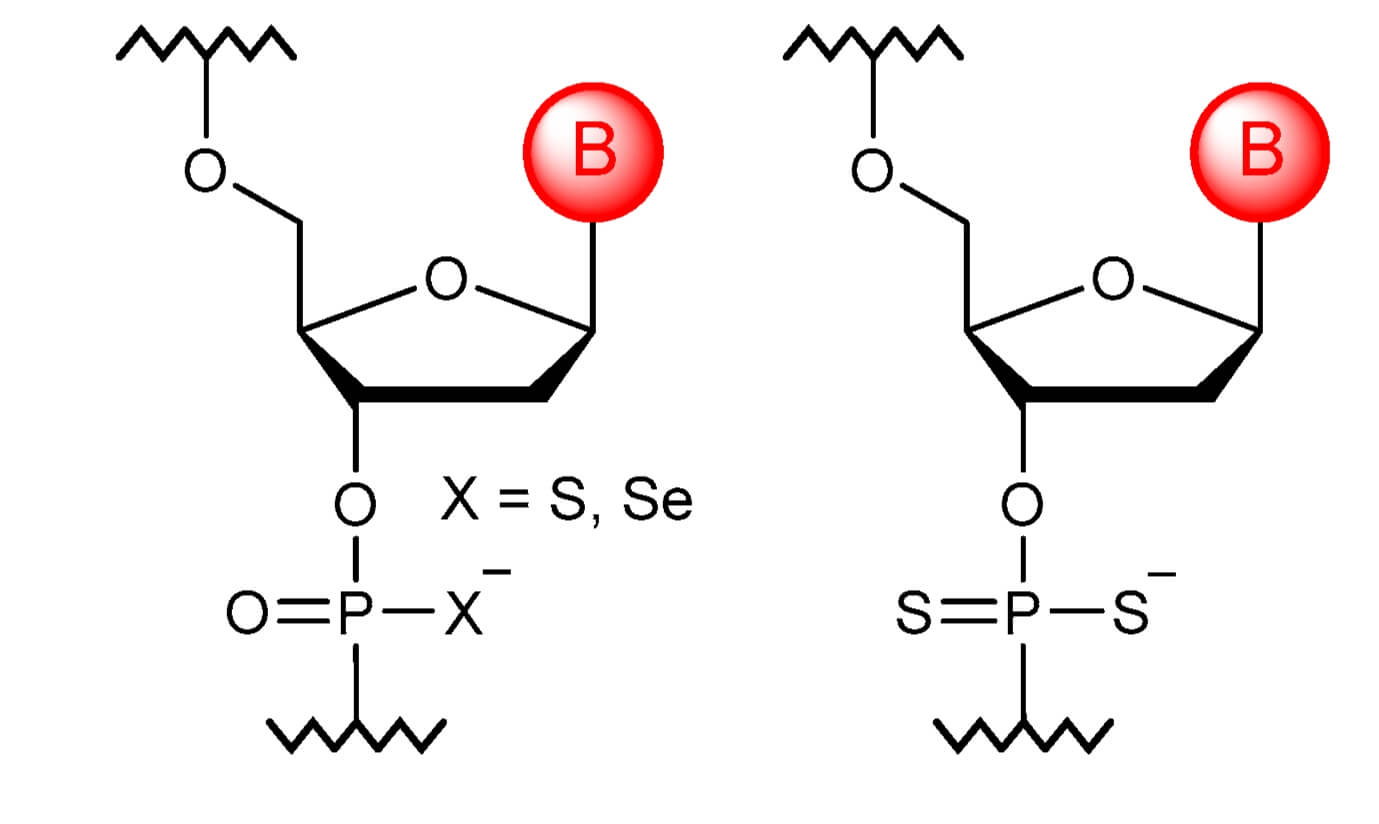 Fig.1 The monomer structures of thiophosphates and dithiophosphates obtained by replacing one or two non-bridging oxygen atoms with sulfur atoms.1
Fig.1 The monomer structures of thiophosphates and dithiophosphates obtained by replacing one or two non-bridging oxygen atoms with sulfur atoms.1
Phosphorothioate modification, one of the most commonly used oligonucleotide chemical modifications, involves substituting a non-bridging oxygen in the natural oligonucleotide's phosphodiester bond (-O-P-O-) with a sulfur atom to form a phosphorothioate bond (-O-P-S-). This seemingly minor structural adjustment significantly improves the oligonucleotide's biological properties. While retaining the negative charge of natural nucleotides (making them the closest analogues), it confers distinct advantages, leading to wide applications in research, diagnostics, and therapeutic fields.
Key Advantages
- Enhanced Nuclease Resistance: Sulfur-induced steric hindrance and electronic changes boost resistance to exo/endonucleases, preserving structure in biological environments, extending half-life, and sustaining activity.
- Improved Pharmacokinetic Profile: Increased plasma protein binding, reduced renal clearance, and enhanced bioavailability; stability enables less frequent dosing, aiding compliance.
- Preserved Biological Activity: Maintains specific base-pairing with target nucleic acids, ensuring efficacy in gene silencing, targeted binding, etc.
- Immunogenicity Regulation: Moderate modification reduces immune overactivation; some promote cellular uptake via binding to specific proteins (e.g., cell surface receptors).
Considerations for Optimal Design
- Optimization of Modification Degree and Mode: Adjust modification range (full/partial) by application: excess reduces targeting; insufficiency limits stability. Design modes (e.g., 3'/5' end modification, intermittent phosphorothioate insertion) to balance stability and targeting.
- Control of Non-Specific Interactions: Modification may bind non-specifically to endogenous components (heparin-like proteins, etc.) via enhanced charge/altered backbone, raising off-target risk. Use sequence optimization and site screening to reduce such interactions, ensuring efficacy and specificity.
- Balance Between Immunogenicity and Toxicity: Moderate modification reduces immunogenicity; high proportions may activate innate immunity, causing inflammation/toxicity. Determine optimal ratio via gradient experiments to avoid overactive immune pathways.
- Synthesis and Purity Control: Phosphorothioate bonds increase synthesis difficulty, with diastereoisomer impurities affecting homogeneity/activity. Use high-precision synthesis and strict purification for accurate modification and compliant impurities.
Workflow
-
Required Starting Materials
- Target Gene/RNA Sequence: The specific genetic target (e.g., mRNA sequence for ASO/siRNA) you aim to modulate.
- Desired Oligonucleotide Sequence: The proposed sequence of your therapeutic oligonucleotide.
- Specific Therapeutic Application Details: Information regarding the disease area, intended delivery route, and desired in vivo stability.
-
Consultation & Design Optimization
Experts collaborate to understand goals, design optimal phosphorothioate strategies (considering placement, density, off-target risks), tailoring for stability, efficacy, and minimal side effects.
-
High-Quality Oligonucleotide Synthesis
State-of-the-art automated platforms precisely synthesize custom oligonucleotides with phosphorothioate linkages, ensuring high purity and yield via robust protocols.
-
Rigorous Quality Control & Purification
Stringent QC (MS, HPLC) verifies sequence integrity, purity, and modification incorporation; advanced purification removes impurities for top-quality products.
-
Comprehensive Biophysical & Biochemical Characterization
In-depth studies (nuclease stability, Tm analysis, protein binding) evaluate PS modification impacts, providing insights into in vivo behavior.
-
Final Deliverables
- Comprehensive Synthesis Report: Detailed documentation of the synthesis process, including reaction parameters and raw data.
- Analytical Data Package: Full characterization data (e.g., MS, HPLC chromatograms, purity analysis, nuclease stability profiles).
- Optimized Oligonucleotide Product: The high-purity, PS-modified oligonucleotide is ready for your research or preclinical studies.
-
Estimated Timeframe Our Phosphorothioate Modification service typically takes 6-12 weeks, depending on sequence complexity, synthesis scale, and characterization depth. Expedited options may be available.
What We Can Offer
Customized Phosphorothioate Design & Synthesis
Tailored modification strategies for therapeutic oligonucleotides, meeting unique stability/specificity needs for optimal application performance.
Scalable Production Capabilities
Flexible volumes from research-grade to large-scale industrial synthesis, supporting all project stages (discovery to preclinical).
Advanced Characterization & Quality Assurance
Comprehensive analytics (nuclease stability, Tm, purity) with robust quality systems, ensuring integrity and efficacy.
Mitigation of Off-Target Interactions
Specialized design to enhance stability while minimizing non-specific binding to endogenous components, boosting specificity and safety.
[Experience the Creative Biolabs Advantage - Get a Quote Today]
FAQs
Q: What are the specific differences in serum stability and cellular uptake efficiency between fully phosphorothioate-modified and end locally modified (e.g., 3'-only/5'-only) oligonucleotides?
A: Fully modified ones have 2-5 times longer serum half-life but may reduce targeted uptake due to excessive negative charge, enhancing non-specific binding to cell surface proteins. Terminal modifications (especially 3') maintain nuclease resistance, retain more natural backbone properties, and suit precise targeting (e.g., ASOs for specific mRNA).
Q: What buffer system and temperature are optimal for long-term (>6 months) storage of phosphorothioate-modified oligonucleotides, and why?
A: Store in 10 mM Tris-HCl (pH 7.5) without EDTA at -80°C in aliquots. Phosphorothioate bonds hydrolyze in acidic conditions; EDTA may chelate trace metals (promoting oxidation). Low temperature slows sulfur oxidation (avoids sulfoxide derivatives).
Q: Can Creative Biolabs help optimize the placement of phosphorothioate modifications in my oligonucleotide sequence?
A: Yes. Experts use oligonucleotide chemistry/biology expertise to optimize modification placement and density, balancing stability/efficacy and minimizing off-target effects for your application.
[Contact Our Team for More Information and to Discuss Your Project]
References
- Novikova, Daria, et al. "A visual compendium of principal modifications within the nucleic acid sugar phosphate backbone." Molecules 29.13 (2024): 3025. DOI: 10.1038/s41467-022-31636-2. DOI: 10.3390/molecules29133025. Distributed under Open Access license CC BY 4.0, A part of the picture was cropped.
