Cell Glycoengineering by Manipulating Sialylation
Role of Sialylation on Therapeutic Glycoproteins
Sialic acids are frequently found as the terminal monosaccharides in the glycoconjugates present on cell surfaces. Therapeutic glycoproteins lacking sialylation experience shorter circulatory half-lives, as the terminal sialic acids mask terminal Gal from recognition by hepatocyte asialoglycoprotein receptors, preventing rapid removal from circulation. Insufficient sialic acid content also introduces significant glycan heterogeneity, leading to inconsistency in the pharmacodynamics of glycoprotein biologics. Furthermore, sialic acid exerts a crucial influence on the activity of antibodies. The presence of α-2,6 sialylation, as opposed to α-2,3 sialic acids, has been noted to influence the anti-inflammatory activity of antibodies. Maximizing sialylation of monoclonal antibodies (mAbs) emerges as an effective strategy to enhance their anti-inflammatory activity. Therefore, it is often a priority to optimize the terminal sialic acids of glycoproteins, which ensures their quality, consistency, and effectiveness as therapeutic agents.
Cell Glycoengineering by Manipulating Sialylation at Creative Biolabs
Sialylation on the glycans of glycol-based therapeutics plays a pivotal role in extending their circulatory lifespan and achieving optimal therapeutic efficacy. Powered by advanced glycoengineering technology and a professional technical team, Creative Biolabs provides comprehensive glycoengineering services by manipulating sialylation utilizing a variety of genetic strategies across different Glycoengineered Cell Lines. Our services are designed to ensure high-quality glycoengineering solutions tailored to our client's needs.
Our Strategies for Manipulating Sialylation
Increasing α-2,6 sialylation
Producing antibodies with α-2,6 sialylation presents a challenge due to the absence of α-2,6 sialyltransferase (α2,6-SiaT) in many expression systems. To address this, we have developed various genetic approaches to successfully engineer cell lines for the production of glycoproteins containing α-2,6 sialylation.
With Overexpression of α2,6-SiaT, we efficiently elevate the sialylation levels of the recombinant antibodies. Combined with stable knockout of α2,3-SiaT (ST3GAL4 and ST3GAL6) by CRISPR/Cas9, antibodies or recombinant proteins with exclusively α-2,6 sialylation can be generated.
Increasing the sialic acid content
Besides overexpression of sialyltransferase gene, elevated sialic acid levels can be achieved by the modulation of genes involved in preceding steps of the glycosylation pathway or by inhibiting genes responsible for encoding sialidases.
For instance, co-overexpression of α2,6-SiaT and β1,4-GalT notably increases the sialic acid content in target proteins. By overexpressing genes implicated in sialic acid biosynthesis and transportation, such as CMP sialic acid synthetase and CMP-sialic acid transporter, enhanced sialic acid content is attainable. Furthermore, we perform efficient RNA-mediated suppression of sialidase to prevent the removal of sialic acid residues from glycoproteins.
Advantages
-
Optional glycoengineering strategies for manipulating sialylation
-
Multiple and advanced techniques for gene regulation
-
Highly efficient knockout by CRISPR/Cas9 and RNA-mediated knockdown
-
High-level expression systems for glycoproteins with elevated sialylation
Published Data
Technology: Antibody glycoengineering
Journal: Frontiers in Immunology
IF: 8.787
Published: 2018
Results: Sialic acid has a manifold impact on the pharmacokinetics of recombinant glycoproteins, as well as a specific impact on bioactivity in ADCC, IVIG, and ADCs. Genetic manipulation of sialyltransferases or targeting enzymes and transporters in the sialic acid biosynthetic pathway can increase the overall sialylation of therapeutic glycoproteins.
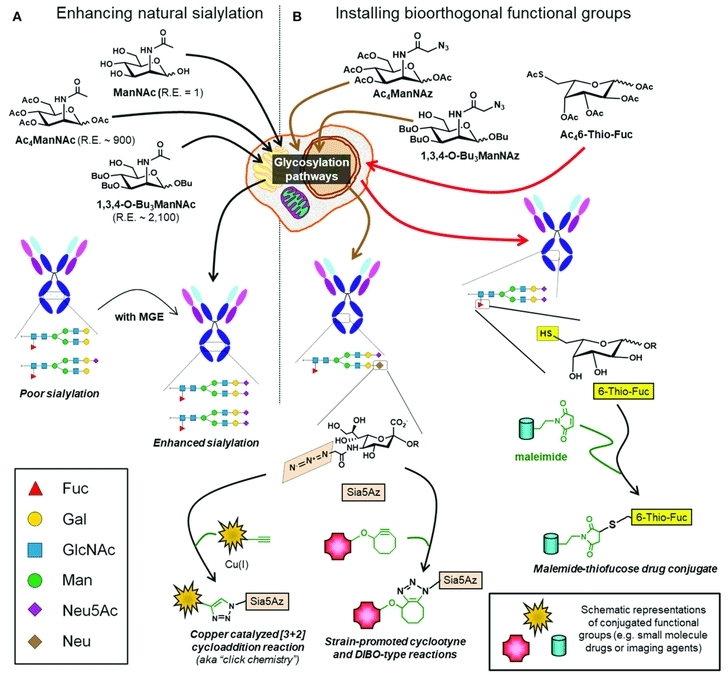 Fig.1 Glycoengineering of mAbs for enhanced sialylation.1, 2
Fig.1 Glycoengineering of mAbs for enhanced sialylation.1, 2
With extensive knowledge and years of experience in genetic editing and glycoengineering, Creative Biolabs is proud to offer comprehensive cell glycoengineering services by manipulating sialylation based on specific requirements. Please feel free to contact us for more details.
References
-
Buettner, Matthew J., et al. "Improving immunotherapy through glycodesign." Frontiers in immunology 9 (2018): 2485.
-
Under Open Access license CC BY 4.0, without modification.
For Research Use Only.
Related Services

 Fig.1 Glycoengineering of mAbs for enhanced sialylation.1, 2
Fig.1 Glycoengineering of mAbs for enhanced sialylation.1, 2

